Chemistry:Potassium trifluoroacetate
From HandWiki
| |
| |
| Identifiers | |
|---|---|
| Properties | |
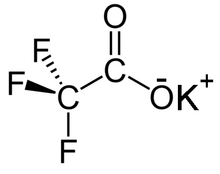
| CF3COOK | |
| Boiling point | 145 °C (418 K)[1] |
| Related compounds | |
Other anions
|
Potassium difluorobromoacetate |
Other cations
|
Sodium trifluoroacetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium trifluoroacetate is the trifluoroacetate salt of potassium, with the chemical formula CF3COOK. It can form an acid salt KH(CF3COO)2.[2]
Preparation and properties
Potassium trifluoroacetate can be obtained by reacting trifluoroacetic acid with potassium hydroxide, potassium carbonate or potassium bicarbonate.
- CF
3COOH + KOH → CF
3COOK + H
2O
It can decompose when heated and reaches the maximum decomposition rate at 220 °C. The products are potassium fluoride and some volatile products, such as carbon dioxide, carbon monoxide, trifluoroacetyl fluoride, etc.[3]
References
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedR - ↑ A. L. Macdonald, J. C. Speakman, D. Hadži (1972). "Crystal structures of the acid salts of some monobasic acids. Part XIV. Neutron-diffraction studies of potassium hydrogen bis(trifluoroacetate) and potassium deuterium bis(trifluoroacetate): crystals with short and symmetrical hydrogen bonds" (in en). J. Chem. Soc., Perkin Trans. 2 (7): 825–832. doi:10.1039/P29720000825. ISSN 0300-9580. http://xlink.rsc.org/?DOI=P29720000825. Retrieved 2019-03-22.
- ↑ M. J. Baillie, D. H. Brown, K. C. Moss, D. W. A. Sharp (1968). "Anhydrous metal trifluoroacetates" (in en). Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 3110. doi:10.1039/j19680003110. ISSN 0022-4944. http://xlink.rsc.org/?DOI=j19680003110. Retrieved 2019-03-22.
 |

