Organization:Laboratory experiments of speciation
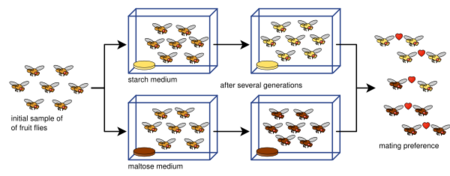
Laboratory experiments of speciation have been conducted for all four modes of speciation: allopatric, peripatric, parapatric, and sympatric; and various other processes involving speciation: hybridization, reinforcement, founder effects, among others. Most of the experiments have been done on flies, in particular Drosophila fruit flies.[1] However, more recent studies have tested yeasts, fungi, and even viruses.
It has been suggested that laboratory experiments are not conducive to vicariant speciation events (allopatric and peripatric) due to their small population sizes and limited generations.[2] Most estimates from studies of nature indicate that speciation takes hundreds of thousands to millions of years.[3] On the other hand, many species are thought to have speciated faster and more recently, such as the European flounders (Platichthys flesus) that spawn in pelagic and demersal zones—having allopatrically speciated in under 3000 generations.[4]
Table of experiments
Six publications have attempted to compile, review, and analyze the experimental research on speciation:
- John Ringo, David Wood, Robert Rockwell, and Harold Dowse in 1985;[5]
- William R. Rice and Ellen E. Hostert in 1993;[6]
- Ann-Britt Florin and Anders Ödeen in 2002;[2]
- Mark Kirkpatrick and Virginie Ravigné in 2002;[7]
- Jerry A. Coyne and H. Allen Orr in 2004;[1] and
- James D. Fry in 2009.[8]
The table summarizes the studies and data reviewed in these publications. It also references several contemporary experiments and is non-exhaustive. In the table, multiple numbers separated by semi-colons in the generations column indicate that multiple experiments were conducted. The replications (in parentheses) indicates the number of populations used in the experiments—i.e. how many times the experiment was replicated. Various types of selection have been imposed on experimental populations and are indicated by the selection type column. Negative or positive results of each experiment are provided by the reproductive isolation column. Pre-zygotic reproductive isolation means that the reproducing individuals in the populations were unable to produce offspring (effectively a positive result). Post-zygotic isolation means that the reproducing individuals were able to produce offspring but they were either sterile or inviable (a positive result as well). Negative results are indicated by "none"—that is, the experiments did not result in reproductive isolation.
| Species | Trait | Generations (replications) [duration] | Tested | Selection type | Studied genetic drift | Reproductive isolation | Reference | Year |
|---|---|---|---|---|---|---|---|---|
| Drosophila melanogaster | Escape response | 18 | Vicariant, reinforcement, parapatric/
sympatric |
Indirect; divergent | Yes | Pre-zygotic | Grant & Mettler[9] | 1969 |
| D. melanogaster | Locomotion | 112 | Vicariant | Indirect; divergent | No | Pre-zygotic | Burnet & Connolly[10] | 1974 |
| D. melanogaster | Temperature, humidity | 70–130 | Vicariant | Indirect; divergent | Yes | Pre-zygotic | Kilias et al.[11] | 1980 |
| D. melanogaster | DDT adaptation | 600 [25 years, +15 years] | Vicariant | Direct | No | Pre-zygotic | Boake et al.[12] | 2003 |
| D. melanogaster | 17, 9, 9, 1, 1, 7, 7, 7, 7 | Vicariant; parapatric/
sympatric |
Direct, divergent | Pre-zygotic in vicariance; none with gene flow | Barker & Karlsson[13] | 1974 | ||
| D. melanogaster | 40; 50 | Reinforcement | Direct; divergent | Pre-zygotic | Crossley[14] | 1974 | ||
| D. melanogaster | Locomotion | 45 | Vicariant | Direct; divergent | No | None | van Dijken & Scharloo[15][16] | 1979 |
| D. melanogaster | Reinforcement | Direct; divergent | Pre-zygotic | Wallace[17] | 1953 | |||
| D. melanogaster | 36; 31 | Reinforcement | Direct; divergent | Pre-zygotic | Knight[18] | 1956 | ||
| D. melanogaster | EDTA adaptation | 25, 25, 25, 14 | Semi-allopatric, reinforcement | Indirect; divergent | No | Post-zygotic | Robertson[19][20] | 1966 |
| D. melanogaster | 25 (8) | Vicariant; reinforcement; parapatric; sympatric | Direct | None | Hostert[21] | 1997 | ||
| D. melanogaster | Abdominal chaeta
number |
21–31 | Vicariant | Direct | Yes | None | Santibanez & Waddington[22] | 1958 |
| D. melanogaster | Sternopleural chaeta number | 32 | Vicariant, reinforcement, parapatric/
sympatric |
Direct | No | None | Barker & Cummins[23] | 1969 |
| D. melanogaster | Phototaxis, geotaxis | 20 | Vicariant | No | None | Markow[24][25] | 1975; 1981 | |
| D. melanogaster | Peripatric | Yes | Rundle et al.[26] | 1998 | ||||
| D. melanogaster | Vicariant; peripatric | Yes | Mooers et al.[27] | 1999 | ||||
| D. melanogaster | 12 | Reinforcement | Divergent | Pre-zygotic | Thoday & Gibson[28] | 1962 | ||
| D. melanogaster | None | Thoday & Gibson[29][30] | 1970; 1971 | |||||
| D. melanogaster | 16 | Reinforcement | Indirect | None | Spiess & Wilke[31] | 1954 | ||
| D. melanogaster | Reinforcement | Direct; divergent | Pre-zygotic | Ehrman[32][33][34][35] | 1971; 1973; 1979; 1983 | |||
| D. melanogaster | Sternopleural chaeta number | 5; 27; 27; 1; 1; 1; 1; 1 | Parapatric/
sympatric |
None | Chabora[36] | 1968 | ||
| D. melanogaster | None | Scharloo[37] | 1967 | |||||
| D. melanogaster | 1, 1 | Coyne & Grant[38] | 1972 | |||||
| D. melanogaster | 25 | Rice[39] | 1985 | |||||
| D. melanogaster | 25 | Disruptive | Pre-zygotic | Rice & Salt[40] | 1988 | |||
| D. melanogaster | 35; 35 | Sympatric | Pre-zygotic | Rice & Salt[41] | 1990 | |||
| D. melanogaster | NaCl and CuSO4 levels in food | [3 years in allopatry, 1 in sympatry] | Allopatric; reinforcement; sympatric | Pre-zygotic in allopatry, none in sympatry | Wallace[42] | 1982 | ||
| D. melanogaster | Reinforcement | Ehrman et al.[43] | 1991 | |||||
| D. melanogaster | Reinforcement | Fukatami & Moriwaki[44] | 1970 | |||||
| Drosophila simulans | Scutellar bristles, development speed, wing width; desiccation resistance, fecundity, ethanol resistance; courtship display, re-mating speed, lek behavior; pupation height, clumped egg laying, general activity | [3 years] | Vicariant; peripatric | Yes | Post-zygotic | Ringo et al.[5] | 1985 | |
| Drosophila paulistorum | 131; 131 | Reinforcement | Direct | Pre-zygotic | Dobzhansky et al.[45] | 1976 | ||
| D. paulistorum | [5 years] | Vicariant | Dobzhansky and Pavlovsky[46] | 1966 | ||||
| Drosophila willistoni | pH adaptation | 34–122 | Vicariant | Indirect; divergent | No | Pre-zygotic | Kalisz & Cordeiro[47] | 1980 |
| Drosophila pseudoobscura | Carbohydrate source | 12 | Vicariant | Indirect | Yes | Pre-zygotic | Dodd[48] | 1989 |
| D. pseudoobscura | Temperature adaptation | 25–60 | Vicariant | Direct | Ehrman[49][50][51][52][53] | 1964;
1969 | ||
| D. pseudoobscura | Phototaxis, geotaxis | 5–11 | Vicariant | Indirect | No | Pre-zygotic | del Solar[54] | 1966 |
| D. pseudoobscura | Vicariant; peripatric | Pre-zygotic | Powell[55][56] | 1978; 1985 | ||||
| D. pseudoobscura | Peripatric; vicariant | Yes | Galiana et al.[57] | 1993 | ||||
| D. pseudoobscura | Temperature photoperiod; food | 37 (78) [33–34 months] | Vicariant | Divergent | Yes | None | Rundle[58] | 2003 |
| D. pseudoobscura & | 22; 16; 9 | Reinforcement | Direct; divergent | Pre-zygotic | Koopman[59] | 1950 | ||
| D. pseudoobscura &
D. persimilis |
18 (4) | Direct | Pre-zygotic | Kessler[60] | 1966 | |||
| Drosophila mojavensis | 12 | Direct | Pre-zygotic | Koepfer[61] | 1987 | |||
| D. mojavensis | Development time | 13 | Divergent | Yes | None | Etges[62] | 1998 | |
| Drosophila adiastola | Peripatric | Yes | Pre-zygotic | Arita & Kaneshiro[63] | 1974 | |||
| Drosophila silvestris | Peripatric | Yes | Ahearn[64] | 1980 | ||||
| Musca domestica | Geotaxis | 38 | Vicariant | Indirect | No | Pre-zygotic | Soans et al.[65] | 1974 |
| M. domestica | Geotaxis | 16 | Vicariant | Direct; divergent | No | Pre-zygotic | Hurd & Eisenburg[66] | 1975 |
| M. domestica | Peripatric | Yes | Meffert & Bryant[67] | 1991 | ||||
| M. domestica | Regan et al.[68] | 2003 | ||||||
| Bactrocera cucurbitae | Development time | 40–51 | Divergent | Yes | Pre-zygotic | Miyatake & Shimizu[69] | 1999 | |
| Zea mays | 6; 6 | Reinforcement | Direct; divergent | Pre-zygotic | Paterniani[70] | 1969 | ||
| Drosophila grimshawi | Peripatric | Jones, Widemo, & Arrendal[71] | N/A | |||||
| Saccharomyces cerevisiae | Leu & Murry[72] | 2006 | ||||||
| D. melanogaster | Reinforcement | Harper & Lambert[73] | 1983 | |||||
| Tribolium castaneum | Pupal weight | 15 (6) | Disruptive | Halliburton & Gall[74] | 1983 | |||
| D. melanogaster | Geotaxis | Divergent | Lofdahl et al.[75] | 1992 | ||||
| D. pseudoobscura | [10 years] | Moya et al.[76] | 1995 | |||||
| Neurospora | Divergent | Dettman et al.[77] | 2008 | |||||
| S. cerevisiae | 500 | Divergent | Dettman et al.[78] | 2007 | ||||
| Sepsis cynipsea | 35 | Martin & Hosken[79] | 2003 | |||||
| D. melanogaster | Wigby & Chapman[80] | 2006 | ||||||
| D. pseudoobscura | Sexual conflict | 48–52 (4; 4; 4) | Bacigalupe et al.[81] | 2007 | ||||
| D. serrata | Rundle et al.[82] | 2005 | ||||||
| Drosophila serrata & D. birchii | Mate recognition | 9 (3; 3) | Reinforcement | Natural | Pre-zygotic | Higgie et al.[83] | 2000 | |
| Enterobacteria phage λ | Escherichia coli receptor exploitation | 35 cycles (6) | Vicariant, sympatric | Pre-zygotic | Meyer et al.[84] | 2016 | ||
| Tetranychus urticae | Resistance to host plant toxin | Overmeer[85] | 1966 | |||||
| T. urticae | Resistance to host plant toxin | Fry[86] | 1999 | |||||
| Helianthus annus × H. petiolaris and H. anomalus | Hybrid | Rieseburg et al.[87] | 1996 | |||||
| S. cerevisiae | 2002 | |||||||
| D. melanogaster | Life history | Ghosh & Joshi[88] | 2012 | |||||
| Drosophila subobscura | Mate behavior | Bárbaro et al.[89] | 2015 | |||||
| Digital organisms | ~42,000; ~850 (20) | Ecological | Post-zygotic | Anderson & Harmon[90] | 2014 | |||
| Schizosaccharomyces pombe | Complete reproductive isolation | Seike et al.[91] | 2015 | |||||
| D. pseudoobscura | Courtship song | 130 | Debelle et al.[92] | 2014 | ||||
| Callosobruchus maculatus | 40 (16) | Debelle et al.[93] | 2010 |
See also
References
- ↑ 1.0 1.1 1.2 Coyne, Jerry A.; Orr, H. Allen (2004), Speciation, Sinauer Associates, pp. 1–545, ISBN 978-0-87893-091-3
- ↑ 2.0 2.1 2.2 Florin, Ann-Britt & Ödeen, Anders (2002), "Laboratory environments are not conducive for allopatric speciation", Journal of Evolutionary Biology 15 (1): 10–19, doi:10.1046/j.1420-9101.2002.00356.x
- ↑ Coyne, Jerry A.; Orr, H. Allen (1997), ""Patterns of Speciation in Drosophila" Revisited", Evolution 51 (1): 295–303, doi:10.1111/j.1558-5646.1997.tb02412.x, PMID 28568795
- ↑ Momigliano, Paolo; Jokinen, Henri; Fraimout, Antoine; Florin, Ann-Britt; Norkko, Alf; Merilä, Juha (2017), "Extraordinarily rapid speciation in a marine fish", PNAS 114 (23): 6074–6079, doi:10.1073/pnas.1615109114, PMID 28533412, PMC 5468626, Bibcode: 2017PNAS..114.6074M, http://www.pnas.org/content/pnas/114/23/6074.full.pdf
- ↑ 5.0 5.1 Ringo, John; Wood, David; Rockwell, Robert; Dowse, Harold (1985), "An Experiment Testing Two Hypotheses of Speciation", The American Naturalist 126 (5): 642–661, doi:10.1086/284445
- ↑ 6.0 6.1 Rice, William R. & Hostert, Ellen E. (1993), "Laboratory Experiments on Speciation: What Have We Learned in 40 Years?", Evolution 47 (6): 1637–1653, doi:10.1111/j.1558-5646.1993.tb01257.x, PMID 28568007
- ↑ 7.0 7.1 Kirkpatrick, Mark & Ravigné, Virginie (2002), "Speciation by Natural and Sexual Selection: Models and Experiments", The American Naturalist 159: S22–S35, doi:10.1086/338370, PMID 18707367
- ↑ 8.0 8.1 Fry, James D. (2009). Laboratory Experiments on Speciation. In Garland, Theodore & Rose, Michael R. "Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments". Pp. 631–656. doi:10.1525/california/9780520247666.003.0020
- ↑ Grant, B. S. & Mettler, L. E. (1969), "Disruptive and stabilizing selection on the" escape" behavior of Drosophila melanogaster", Genetics 62 (3): 625–637, doi:10.1093/genetics/62.3.625, PMID 17248452
- ↑ Burnet, B. & Connolly, K. (1974). Activity and sexual behavior in Drosophila melanogaster. In Abeelen, J. H. V. F. (eds). The Genetics of Behaviour. North-Holland, Amsterdam. Pp. 201–258.
- ↑ Kilias, G., Alahiotis, S. N., & Pelecanos, M. (1980), "A Multifactorial Genetic Investigation of Speciation Theory Using Drosophila melanogaster", Evolution 34 (4): 730–737, doi:10.2307/2408027, PMID 28563991
- ↑ Boake, C. R. B., Mcdonald, K., Maitra, S., Ganguly, R. (2003), "Forty years of solitude: life-history divergence and behavioural isolation between laboratory lines of Drosophila melanogaster", Journal of Evolutionary Biology 16 (1): 83–90, doi:10.1046/j.1420-9101.2003.00505.x, PMID 14635883
- ↑ Barker, J. S. F. & Karlsson, L. J. E. (1974), "Effects of population size and selection intensity on responses to disruptive selection in Drosophila melanogaster", Genetics 78 (2): 715–735, doi:10.2307/2407287, PMID 4217303
- ↑ Crossley, Stella A. (1974), "Changes in Mating Behavior Produced by Selection for Ethological Isolation Between Ebony and Vestigial Mutants of Drosophila melanogaster", Evolution 28 (4): 631–647, doi:10.1111/j.1558-5646.1974.tb00795.x, PMID 28564833
- ↑ van Dijken, F. R. & Scharloo, W. (1979), "Divergent selection on locomotor activity in Drosophila melanogaster. I. Selection response", Behavior Genetics 9 (6): 543–553, doi:10.1007/BF01067350, PMID 122270
- ↑ van Dijken, F. R. & Scharloo, W. (1979), "Divergent selection on locomotor activity in Drosophila melanogaster. II. Test for reproductive isolation between selected lines", Behavior Genetics 9 (6): 555–561, doi:10.1007/BF01067351, PMID 122271
- ↑ Wallace, B. (1953), "Genetic divergence of isolated populations of Drosophila melanogaster", Proceedings of the Ninth International Congress of Genetics 9: 761–764
- ↑ Knight, G. R., Robertson, Alan, & Waddington, C. H. (1956), "Selection for sexual isolation within a species", Evolution 10 (1): 14–22, doi:10.1111/j.1558-5646.1956.tb02825.x
- ↑ Robertson, Forbes W. (1966), "A test of sexual isolation in Drosophila", Genetical Research 8 (2): 181–187, doi:10.1017/S001667230001003X, PMID 5922518
- ↑ Robertson, Forbes W. (1966), "The ecological genetics of growth in Drosophila 8. Adaptation to a New Diet", Genetical Research 8 (2): 165–179, doi:10.1017/S0016672300010028, PMID 5922517
- ↑ Hostert, Ellen E. (1997), "Reinforcement: a new perspective on an old controversy", Evolution 51 (3): 697–702, doi:10.1111/j.1558-5646.1997.tb03653.x, PMID 28568598
- ↑ Koref Santibañez, S. & Waddington, C. H. (1958), "The origin of sexual isolation between different lines within a species", Evolution 12 (4): 485–493, doi:10.2307/2405959
- ↑ Barker, J. S. F. & Cummins, L. J. (1969), "The effect of selection for sternopleural bristle number in mating behaviour in Drosophila melanogaster", Genetics 61 (3): 713–719, doi:10.1093/genetics/61.3.713, PMID 17248436
- ↑ Markow, Therese Ann (1975), "A genetic analysis of phototactic behavior in Drosophila melanogaster", Genetics 79 (3): 527–534, doi:10.1093/genetics/79.3.527, PMID 805084
- ↑ Markow, Therese Ann (1981), "Mating preferences are not predictive of the direction of evolution in experimental populations of Drosophila", Science 213 (4514): 1405–1407, doi:10.1126/science.213.4514.1405, PMID 17732575, Bibcode: 1981Sci...213.1405M
- ↑ Rundle, H. D., Mooers, Arne Ø. & Whitlock, Michael C. (1998), "Single founder-flush events and the evolution of reproductive isolation", Evolution 52 (6): 1850–1855, doi:10.1111/j.1558-5646.1998.tb02263.x, PMID 28565304
- ↑ Mooers, Arne Ø., Rundle, Howard D. & Whitlock, Michael C. (1999), "The effects of selection and bottlenecks on male mating success in peripheral isolates", American Naturalist 153 (4): 437–444, doi:10.1086/303186, PMID 29586617
- ↑ Thoday, J. M. & Gibson, J. B. (1962), "Isolation by disruptive selection", Nature 193 (4821): 1164–1166, doi:10.1038/1931164a0, PMID 13920720, Bibcode: 1962Natur.193.1164T
- ↑ Thoday, J. M. & Gibson, J. B. (1970), "The probability of isolation by disruptive selection", Nature 104 (937): 219–230, doi:10.1086/282656
- ↑ Scharloo, W. (1971), "Reproductive isolation by disruptive selection: Did it occur?", American Naturalist 105 (941): 83–86, doi:10.1086/282706
- ↑ Spiess, E. B. & Wilke, C. M. (1984), "Still another attempt to achieve assortive mating by disruptive selection in Drosophila", Evolution 38 (3): 505–515, doi:10.2307/2408700, PMID 28555983
- ↑ Ehrman, Lee (1971), "Natural selection and the origin of reproductive isolation", American Naturalist 105 (945): 479–483, doi:10.1086/282739
- ↑ Ehrman, Lee (1973), "More on natural selection and the origin of reproductive isolation", American Naturalist 107 (954): 318–319, doi:10.1086/282835
- ↑ Ehrman, Lee (1979), "Still more on natural selection and the origin of reproductive isolation", American Naturalist 113 (1): 148–150, doi:10.1086/283371
- ↑ Ehrman, Lee (1983), "Fourth report on natural selection for the origin of reproductive isolation", American Naturalist 121 (2): 290–293, doi:10.1086/284059
- ↑ Chabora, Alice J. (1968), "Disruptive selection for sternopleural chaeta number in various strains of Drosophila melanogaster", American Naturalist 102 (928): 525–532, doi:10.1086/282565
- ↑ Scharloo, W., Hoogmoed, M. S. & Kuile, A. T. (1967), "Stabilizing and disruptive selection on a mutant character in Drosophila. I. The phenotypic variance and its components.", Genetics 56 (4): 709–726, doi:10.1093/genetics/56.4.709, PMID 6061662
- ↑ Coyne, Jerry A. & and Grant, Bruce (1972), "Disruptive selection on I-maze activity in Drosophila melanogaster", Genetics 71 (1): 185–188, doi:10.1093/genetics/71.1.185, PMID 17248572
- ↑ Rice, W. R. (1985), "Disruptive selection on habitat preference and the evolution of reproductive isolation: an exploratory experiment", Evolution 39 (3): 645–656, doi:10.1111/j.1558-5646.1985.tb00401.x, PMID 28561974
- ↑ Rice, William R. & Salt, George, W. (1988), "Speciation via disruptive selection on habitat preference", American Naturalist 131 (6): 911–917, doi:10.1086/284831
- ↑ Rice, William R. & Salt, George, W. (1990), "The evolution of reproductive isolation as a correlated character under sympatric conditions: experimental evidence", Evolution 44 (5): 1140–1152, doi:10.2307/2409278, PMID 28563894
- ↑ Wallace, B. (1982), "Drosophila melanogaster populations selected for resistances to NaCl and CuSO4 in both allopatry and sympatry", Journal of Heredity 73 (1): 35–42, doi:10.1093/oxfordjournals.jhered.a109572, PMID 6802898
- ↑ Ehrman, Lee, White, Marney A. & Wallace, B. (1991). A long-term study involving Drosophila melanogaster and toxic media. In Hecht, M. K., Wallace, B., & Maclntyre, R. J. (eds). Evolutionary biology, vol. 25. Plenum Press, New York. Pp. 175–209
- ↑ Fukatami, A & Moriwaki, D. (1970), "Selection for sexual isolation in Drosophila melanogaster by a modification of Koopman's method", The Japanese Journal of Genetics 45 (3): 193–204, doi:10.1266/jjg.45.193
- ↑ Dobzhansky, Theodosius; Pavlovsky, O.; Powell, J. R. (1976), "Partially Successful Attempt to Enhance Reproductive Isolation Between Semispecies of Drosophila paulistorum", Evolution 30 (2): 201–212, doi:10.2307/2407696, PMID 28563045
- ↑ Dobzhansky, Theodosius & Pavlovsky, O. (1966), "Spontaneous origin of an incipient species in the Drosophila paulistorum complex", Proceedings of the National Academy of Sciences 55 (4): 723–733, doi:10.1073/pnas.55.4.727, PMID 5219677, Bibcode: 1966PNAS...55..727D
- ↑ de Oliveira, Alice Kalisz & Cordeiro, Antonio Rodrigues (1980), "Adaptation of Drosophila willistoni experimental populations to extreme pH medium", Heredity 44: 123–130, doi:10.1038/hdy.1980.11
- ↑ Dodd, Diane M. B. (1989), "Reproductive Isolation as a Consequence of Adaptive Divergence in Drosophila pseudoobscura", Evolution 43 (6): 1308–1311, doi:10.2307/2409365, PMID 28564510
- ↑ Ehrman, Lee (1964), "Genetic divergence in M. Vetukhiv's experimental populations of Drosophila pseudoobscura 1. Rudiments of sexual isolation", Genetical Research 5: 150–157, doi:10.1017/S0016672300001099
- ↑ Mouradael, K. (1965), "Genetic divergence in M. Vetukhiv's experimental populations of Drosophila pseudoobscura 2. Longevity", Genetical Research 6: 139–146, doi:10.1017/S0016672300004006, PMID 14297592
- ↑ Anderson, Wyatt, W. (1966), "Genetic divergence in M. Vetukhiv's experimental populations of Drosophila pseudoobscura 3. Divergence in Body Size", Genetical Research 7 (2): 255–266, doi:10.1017/S0016672300009666
- ↑ Kitagawa, Osamu (1967), "Genetic divergence in M. Vetukhiv's experimental populations of Drosophila pseudoobscura: IV. Relative viability", Genetical Research 10 (7): 303–312, doi:10.1017/S001667230001106X
- ↑ Ehrman, Lee (1969), "Genetic divergence in M. Vetukhiv's experimental populations of Drosophila pseudoobscura. 5. A further study of rudiments of sexual isolation", American Midland Naturalist 82 (1): 272–276, doi:10.2307/2423835
- ↑ del Solar, Eduardo (1966), "Sexual isolation caused by selection for positive and negative phototaxis and geotaxis in Drosophila pseudoobscura", Proceedings of the National Academy of Sciences 56 (2): 484–487, doi:10.1073/pnas.56.2.484, PMID 5229969
- ↑ Powell, Jeffrey R. (1978), "The Founder-Flush Speciation Theory: An Experimental Approach", Evolution 32 (3): 465–474, doi:10.2307/2407714, PMID 28567948
- ↑ Dodd, Diane M. B. & Powell, Jeffrey R. (1985), "Founder-Flush Speciation: An Update of Experimental Results with Drosophila", Evolution 39 (6): 1388–1392, doi:10.1111/j.1558-5646.1985.tb05704.x, PMID 28564258
- ↑ Galiana, Augustí, Moya, Andres & Ayala, Fransisco J. (1993), "Founder-flush speciation in Drosophila pseudoobscura: a large scale experiment", Evolution 47 (2): 432–444, doi:10.1111/j.1558-5646.1993.tb02104.x, PMID 28568735
- ↑ Rundle, Howard D. (2003), "Divergent environments and population bottlenecks fail to generate premating isolation in Drosophila pseudoobscura", Evolution 57 (11): 2557–2565, doi:10.1111/j.0014-3820.2003.tb01499.x, PMID 14686531
- ↑ Koopman, Karl F. (1950), "Natural Selection for Reproductive Isolation Between Drosophila pseudoobscura and Drosophila persimilis", Evolution 4 (2): 135–148, doi:10.2307/2405390
- ↑ Kessler, Seymour (1966), "Selection For and Against Ethological Isolation Between Drosophila pseudoobscura and Drosophila persimilis", Evolution 20 (4): 634–645, doi:10.2307/2406597, PMID 28562900
- ↑ Koepfer, H. Roberta (1987), "Selection for Sexual Isolation Between Geographic Forms of Drosophila mojavensis. I Interactions Between the Selected Forms", Evolution 41 (1): 37–48, doi:10.2307/2408971, PMID 28563762
- ↑ Etges, W. J. (1998), "Premating isolation is determined by larval rearing substrates in cactophilis Drosophila mojavensis. IV. Correlated responses in behavioral isolation to artificial selection on a life-history trait", American Naturalist 152 (1): 129–144, doi:10.1086/286154, PMID 18811406
- ↑ Arita, Lorna H. & Kaneshiro, Kenneth Y. (1979), "Ethological Isolation Between Two Stocks of Drosophila Adiastola Hardy", Hawaiian Entomological Society 23 (1): 31–34
- ↑ Ahearn, J. N. (1980), "Evolution of behavioral reproductive isolation in a laboratory stock of Drosophila silvestris", Experientia 36 (1): 63–64, doi:10.1007/BF02003975
- ↑ Soans, A. Benedict; Pimentel, David; Soans, Joyce S. (1974), "Evolution of Reproductive Isolation in Allopatric and Sympatric Populations", The American Naturalist 108 (959): 117–124, doi:10.1086/282889
- ↑ Hurd, L. E. & Eisenberg, Robert M. (1975), "Divergent Selection for Geotactic Response and Evolution of Reproductive Isolation in Sympatric and Allopatric Populations of Houseflies", The American Naturalist 109 (967): 353–358, doi:10.1086/283002
- ↑ Meffert, L. M. & Bryant, E. H. (1991), "Mating propensity and courtship behavior in serially bottlenecked lines of the housefly", Evolution 45 (2): 293–306, doi:10.1111/j.1558-5646.1991.tb04404.x, PMID 28567864
- ↑ Regan, J. L.; Meffert, L. M.; Bryant, E. H. (2003), "A direct experimental test of founder-flush effects on the evolutionary potential for assortative mating", Journal of Evolutionary Biology 16 (2): 302–312, doi:10.1046/j.1420-9101.2003.00521.x, PMID 14635869
- ↑ Miyatake, Takahisa & Shimizu, Toru (1999), "Genetic correlations between life-history and behavioral traits can cause reproductive isolation", Evolution 53 (1): 201–208, doi:10.2307/2640932, PMID 28565193
- ↑ Paterniani, E. (1969), "Selection for Reproductive Isolation between Two Populations of Maize, Zea mays L.", Evolution 23 (4): 534–547, doi:10.2307/2406851, PMID 28562870
- ↑ Ödeen, Anders & Florin, Ann-Britt (2002), "Sexual selection and peripatric speciation: the Kaneshiro model revisited", Journal of Evolutionary Biology 15 (2): 301–306, doi:10.1046/j.1420-9101.2002.00378.x
- ↑ Leu, J. Y. & Murray, A. W. (2006), "Experimental evolution of mating discrimination in budding yeast", Current Biology 16 (3): 280–286, doi:10.1016/j.cub.2005.12.028, PMID 16461281
- ↑ Harper, A. A. & Lambert, D. M. (1983), "The population genetics of reinforcing selection", Genetica 62 (1): 15–23, doi:10.1007/BF00123305
- ↑ Halliburton, Richard & Gall, G. A. E. (1981), "Disruptive selection and assortative mating in Tribolium castaneum", Evolution 35 (5): 829–843, doi:10.1111/j.1558-5646.1981.tb04947.x, PMID 28581046
- ↑ Lofdahl, L. Katharine; Hu, Dan; Ehrman, Lee; Hirsch, Jerry; Skoog, Linda (1992), "Incipient reproductive isolation and evolution in laboratory Drosophila melanogaster selected for geotaxis", Animal Behaviour 44 (4): 783–786, doi:10.1016/S0003-3472(05)80307-0
- ↑ Moya, A.; Galiana, A.; Ayala, F. J. (1995), "Founder-effect speciation theory: failure of experimental corroboration", Proceedings of the National Academy of Sciences 92 (9): 3983–3986, doi:10.1073/pnas.92.9.3983, PMID 7732017, Bibcode: 1995PNAS...92.3983M
- ↑ Dettman, Jeremy R.; Anderson, James B.; Kohn, Linda M. (2008), "Divergent adaptation promotes reproductive isolation among experimental populations of the filamentous fungus Neurospora", BMC Evolutionary Biology 8 (35): 35, doi:10.1186/1471-2148-8-35, PMID 18237415
- ↑ Dettman, Jeremy R.; Sirjusingh, Caroline; Kohn, Linda M.; Anderson, James B. (2007), "Incipient speciation by divergent adaptation and antagonistic epistasis in yeast", Nature 447 (7144): 585–588, doi:10.1038/nature05856, PMID 17538619, Bibcode: 2007Natur.447..585D
- ↑ Martin, Oliver Y. & Hosken, David J. (2003), "The evolution of reproductive isolation through sexual conflict", Nature 423 (6943): 979–982, doi:10.1038/nature01752, PMID 12827200, Bibcode: 2003Natur.423..979M
- ↑ Wigby, S. & Chapman, T. (2006), "No evidence that experimental manipulation of sexual conflict drives premating reproductive isolation in Drosophila melanogaster", Journal of Evolutionary Biology 19 (4): 1033–1039, doi:10.1111/j.1420-9101.2006.01107.x, PMID 16780504
- ↑ Bacigalupe, L. D.; Crudgington, H. S.; Hunter, F.; Moore, A. J.; Snook, R. R. (2007), "Sexual conflict does not drive reproductive isolation in experimental populations of Drosophila pseudoobscura", Journal of Evolutionary Biology 20 (5): 1763–1771, doi:10.1111/j.1420-9101.2007.01389.x, PMID 17714294
- ↑ Rundle, Howard D.; Chenoweth, Steve F.; Doughty, Paul; Blows, Mark W. (2005), "Divergent selection and the evolution of signal traits and mating preferences", PLOS Biology 3 (11): e368, doi:10.1371/journal.pbio.0030368, PMID 16231971
- ↑ Higgie, Megan; Chenoweth, Steve F.; Blows, Mark W. (2000), "Natural selection and the reinforcement of mate recognition", Science 290 (5491): 519–521, doi:10.1126/science.290.5491.519, PMID 11039933, Bibcode: 2000Sci...290..519H, https://espace.library.uq.edu.au/view/UQ:10714/Higgie_et_al_200.pdf
- ↑ Meyer, Justin R.; Dobias, Devin T.; Medina, Sarah J.; Servilio, Lisa; Gupta, Animesh; Lenski, Richard E. (2016), "Ecological speciation of bacteriophage lambda in allopatry and sympatry", Science 354 (6317): 1301–1304, doi:10.1126/science.aai8446, PMID 27884940, Bibcode: 2016Sci...354.1301M
- ↑ Overmeer, W. P. J. (1966), "Intersterility as a Consequence of Insecticide Selections in Tetranychus urticae Koch (Acari: Tetranychidae)", Nature 209 (321): 321, doi:10.1038/209321a0, PMID 5950361, Bibcode: 1966Natur.209..321O
- ↑ Fry, James D. (1999), "The role of adaptation to host plants in the evolution of reproductive isolation: Negative evidence from Tetranychus urticae Koch", Experimental & Applied Acarology 23 (5): 379–387, doi:10.1023/A:1006245711950
- ↑ Rieseberg, L. H.; Sinervo B.; Linder, C. R.; Ungerer, M.C.; Arias, D. M. (1996), "Role of Gene Interactions in Hybrid Speciation: Evidence from Ancient and Experimental Hybrids", Science 272 (5262): 741–745, doi:10.1126/science.272.5262.741, PMID 8662570, Bibcode: 1996Sci...272..741R
- ↑ Ghosh, Shampa M. & Joshi, Amitabh (2012), "Evolution of reproductive isolation as a by-product of divergent life-history evolution in laboratory populations of Drosophila melanogaster", Ecology and Evolution 2 (12): 3214–3226, doi:10.1002/ece3.413, PMID 23301185
- ↑ Bárbaro, Margarida; Mira, Mário S.; Fragata, Inês; Simões, Pedro; Lima, Margarida; Lopes-Cunha, Miguel; Kellen, Bárbara; Santos, Josiane et al. (2015), "Evolution of mating behavior between two populations adapting to common environmental conditions", Ecology and Evolution 5 (8): 1609–1617, doi:10.1002/ece3.1454, PMID 25937905
- ↑ Anderson, Carlos J. R. & Harmon, Luke (2014), "Ecological and Mutation-Order Speciation in Digital Organisms", The American Naturalist 183 (2): 257–269, doi:10.1086/674359, PMID 24464199
- ↑ Seike, Taisuke; Nakamura, Taro; Shimoda, Chikashi (2015), "Molecular coevolution of a sex pheromone and its receptor triggers reproductive isolation in Schizosaccharomyces pombe", PNAS 112 (14): 4405–4410, doi:10.1073/pnas.1501661112, PMID 25831518, Bibcode: 2015PNAS..112.4405S
- ↑ Debelle, Allan; Ritchie, Michael G.; Snook, Rhonda R. (2014), "Evolution of divergent female mating preference in response to experimental sexual selection", Evolution 68 (9): 2524–2533, doi:10.1111/evo.12473, PMID 24931497
- ↑ Fricke, C; Andersson, C.; Arnqvist, G. (2010), "Natural selection hampers divergence of reproductive traits in a seed beetle", Journal of Evolutionary Biology 23 (9): 1857–1867, doi:10.1111/j.1420-9101.2010.02050.x, PMID 20646133
 |


