Biology:Phototaxis
Phototaxis is a kind of taxis, or locomotory movement, that occurs when a whole organism moves towards or away from a stimulus of light.[1] This is advantageous for phototrophic organisms as they can orient themselves most efficiently to receive light for photosynthesis. Phototaxis is called positive if the movement is in the direction of increasing light intensity and negative if the direction is opposite.[2]
Phototaxis has been described in microorganisms and algea, insects and other invertebrates, and vertebrates. Typically nocturnal insects can show positive phototaxis, while nocturnal mammals often show negative phototaxis.
Phototaxis in bacteria and archea
Template:Microbial and microbot movement
Phototaxis can be advantageous for phototrophic bacteria as they can orient themselves most efficiently to receive light for photosynthesis. Phototaxis is called positive if the movement is in the direction of increasing light intensity and negative if the direction is opposite.[2]
Two types of positive phototaxis are observed in prokaryotes (bacteria and archea). The first is called "scotophobotaxis" (from the word "scotophobia"), which is observed only under a microscope. This occurs when a bacterium swims by chance out of the area illuminated by the microscope. Entering darkness signals the cell to reverse flagella rotation direction and reenter the light. The second type of phototaxis is true phototaxis, which is a directed movement up a gradient to an increasing amount of light. This is analogous to positive chemotaxis except that the attractant is light rather than a chemical.
Phototactic responses are observed in a number of bacteria and archae, such as Serratia marcescens. Photoreceptor proteins are light-sensitive proteins involved in the sensing and response to light in a variety of organisms. Some examples are bacteriorhodopsin and bacteriophytochromes in some bacteria. See also: phytochrome and phototropism.
Most prokaryotes (bacteria and archaea) are unable to sense the direction of light, because at such a small scale it is very difficult to make a detector that can distinguish a single light direction. Still, prokaryotes can measure light intensity and move in a light-intensity gradient. Some gliding filamentous prokaryotes can even sense light direction and make directed turns, but their phototactic movement is very slow. Some bacteria and archaea are phototactic.[3][4][5]
In most cases the mechanism of phototaxis is a biased random walk, analogous to bacterial chemotaxis. Halophilic archaea, such as Halobacterium salinarum, use sensory rhodopsins (SRs) for phototaxis.[6][7] Rhodopsins are 7 transmembrane proteins that bind retinal as a chromophore. Light triggers the isomerization of retinal,[8] which leads to phototransductory signalling via a two-component phosphotransfer relay system. Halobacterium salinarum has two SRs, SRI and SRII, which signal via the transducer proteins Htr1 and Htr2 (halobacterial transducers for SRs I and II), respectively.[9][10] The downstream signalling in phototactic archaebacteria involves CheA, a histidine kinase, which phosphorylates the response regulator, CheY.[11] Phosphorylated CheY induces swimming reversals. The two SRs in Halobacterium have different functions. SRI acts as an attractant receptor for orange light and, through a two-photon reaction, a repellent receptor for near-UV light, while SRII is a repellent receptor for blue light. Depending on which receptor is expressed, if a cell swims up or down a steep light gradient, the probability of flagellar switch will be low. If light intensity is constant or changes in the wrong direction, a switch in the direction of flagellar rotation will reorient the cell in a new, random direction.[12] As the length of the tracks is longer when the cell follows a light gradient, cells will eventually get closer to or further away from the light source. This strategy does not allow orientation along the light vector and only works if a steep light gradient is present (i.e. not in open water).[5]
Some cyanobacteria (e.g. Anabaena, Synechocystis) can slowly orient along a light vector. This orientation occurs in filaments or colonies, but only on surfaces and not in suspension.[13][14] The filamentous cyanobacterium Synechocystis is capable of both positive and negative two-dimensional phototactic orientation. The positive response is probably mediated by a bacteriophytochrome photoreceptor, TaxD1. This protein has two chromophore-binding GAF domains, which bind biliverdin chromophore,[15] and a C-terminal domain typical for bacterial taxis receptors (MCP signal domain). TaxD1 also has two N-terminal transmembrane segments that anchor the protein to the membrane.[16][17][18] The photoreceptor and signalling domains are cytoplasmic and signal via a CheA/CheY-type signal transduction system to regulate motility by type IV pili.[19] TaxD1 is localized at the poles of the rod-shaped cells of Synechococcus elongatus, similarly to MCP containing chemosensory receptors in bacteria and archaea.[20] How the steering of the filaments is achieved is not known. The slow steering of these cyanobacterial filaments is the only light-direction sensing behaviour prokaryotes could evolve owing to the difficulty in detecting light direction at this small scale.[5]
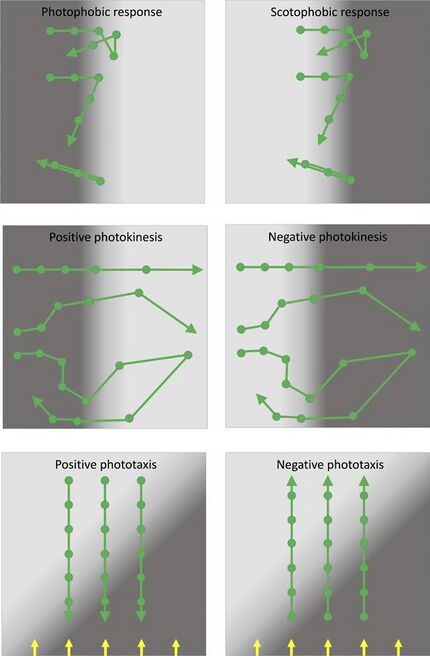
Middle: photokinesis involving changes in speed induced by changing light intensity. In patchy light environments, positive photokinesis results in accumulation in low light areas (and vice versa for negative photokinesis).
Bottom: true phototaxis results in movement towards or away from a light source, but is not a response to a light gradient. Direction of parallel illumination is indicated by the yellow arrows.
The ability to link light perception to control of motility is found in a very wide variety of prokaryotes, indicating that this ability must confer a range of physiological advantages.[22][23] Most directly, the light environment is crucial to phototrophs as their energy source. Phototrophic prokaryotes are extraordinarily diverse, with a likely role for horizontal gene transfer in spreading phototrophy across multiple phyla.[24] Thus, different groups of phototrophic prokaryotes may have little in common apart from their exploitation of light as an energy source, but it should be advantageous for any phototroph to be able to relocate in search of better light environments for photosynthesis. To do this efficiently requires the ability to control motility in response to integrated information on the intensity of light, the spectral quality of light and the physiological status of the cell. A second major reason for light-controlled motility is to avoid light at damaging intensities or wavelengths: this factor is not confined to photosynthetic bacteria since light (especially in the UV region) can be dangerous to all prokaryotes, primarily because of DNA and protein damage [25] and inhibition of the translation machinery by light-generated reactive oxygen species.[26][21]
Finally, light signals potentially contain rich and complex information about the environment, and the possibility should not be excluded that bacteria make sophisticated use of this information to optimize their location and behavior. For example, plant or animal pathogens could use light information to control their location and interaction with their hosts, and in fact light signals are known to regulate development and virulence in several non-phototrophic prokaryotes.[27][28] Phototrophs could also benefit from sophisticated information processing, since their optimal environment is defined by a complex combination of factors including light intensity, light quality, day and night cycles, the availability of raw materials and alternative energy sources, other beneficial or harmful physical and chemical factors and sometimes the presence of symbiotic partners. Light quality strongly influences specialized developmental pathways in certain filamentous cyanobacteria, including the development of motile hormogonia and nitrogen-fixing heterocysts.[29] Since hormogonia are important for establishing symbiotic partnerships between cyanobacteria and plants, and heterocysts are essential for nitrogen fixation in those partnerships, it is tempting to speculate that the cyanobacteria may be using light signals as one way to detect the proximity of a plant symbiotic partner. Within a complex and heterogeneous environment such as a phototrophic biofilm, many factors crucial for growth could vary dramatically even within the limited region that a single motile cell could explore.[30][31] We should therefore expect that prokaryotes living in such environments might control their motility in response to a complex signal transduction network linking a range of environmental cues.[21]
The photophobic response is a change in the direction of motility in response to a relatively sudden increase in illumination: classically, the response is to a temporal change in light intensity, which the bacterium may experience as it moves into a brightly illuminated region. The directional switch may consist of a random selection of a new direction ('tumbling') or it may be a simple reversal in the direction of motility. Either has the effect of repelling cells from a patch of unfavorable light. Photophobic responses have been observed in prokaryotes as diverse as Escherichia coli, purple photosynthetic bacteria and haloarchaea.[32][23][21]
The scotophobic (fear of darkness) response is the converse of the photophobic response described above: a change in direction (tumbling or reversal) is induced when the cell experiences a relatively sudden drop in light intensity. Photophobic and scotophobic responses both cause cells to accumulate in regions of specific (presumably favorable) light intensity and spectral quality. Scotophobic responses have been well documented in purple photosynthetic bacteria, starting with the classic observations of Engelmann in 1883,[33] and in cyanobacteria.[22] Scotophobic/photophobic responses in flagellated bacteria closely resemble the classic 'biased random walk' mode of bacterial chemotaxis, which links perception of temporal changes in the concentration of a chemical attractant or repellent to the frequency of tumbling.[34] The only significant distinction is that the scotophobic/photophobic responses involve perception of temporal changes in light intensity rather than the concentration of a chemical.[21]
Photokinesis is a light-induced change in the speed (but not direction) of movement. Photokinesis may be negative (light-induced reduction of motility) or positive (light-induced stimulation of motility). Photokinesis can cause cells to accumulate in regions of favorable illumination: they linger in such regions or accelerate out of regions of unfavorable illumination. Photokinesis has been documented in cyanobacteria and purple photosynthetic bacteria.[22][21]
True phototaxis consists of directional movement which may be either towards a light source (positive phototaxis) or away from a light source (negative phototaxis). In contrast to the photophobic/scotophobic responses, true phototaxis is not a response to a temporal change in light intensity. Generally, it seems to involve direct sensing of the direction of illumination rather than a spatial gradient of light intensity. True phototaxis in prokaryotes is sometimes combined with social motility, which involves the concerted movement of an entire colony of cells towards or away from the light source. This phenomenon could also be described as community phototaxis. True phototaxis is widespread in eukaryotic green algae,[35] but among the prokaryotes it has been documented only in cyanobacteria,[22][17] and in social motility of colonies of the purple photosynthetic bacterium Rhodocista centenaria.[36][21]
Phototaxis in protists
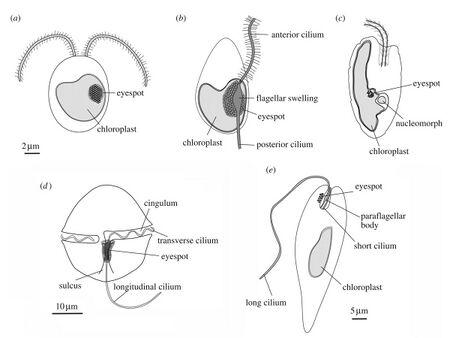
(a) green alga (b) heterokont zoospore (c) cryptomonad alga
(d) dinoflagellate (e) Euglena
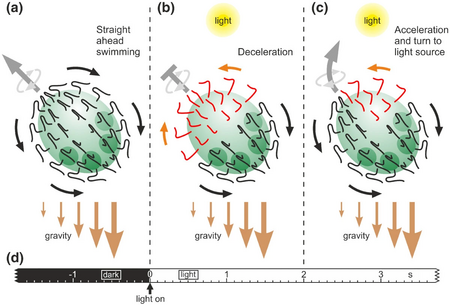
(b) A sudden dark-light switch causes the flagellar beating to reverse in the anterior hemisphere and the deceleration of the spheroid's forward movement (photophobic response)
(c) After approximately 2 seconds, only cells on the illuminated side of the anterior hemisphere of the rotating spheroid show the reversed flagellar beating direction, resulting in an acceleration of the spheroid's forward movement and turning toward the light source. Gravity assists the phototactic movements because it pulls more on the posterior hemisphere due to an anisotropic mass distribution caused by the denser daughter spheroids within the posterior hemisphere and probably also by the closer spacing of the somatic cells in the posterior hemisphere
Some protists (unicellular eukaryotes) can also move toward or away from light, by coupling their locomotion strategy with a light-sensing organ.[38] Eukaryotes evolved for the first time in the history of life the ability to follow light direction in three dimensions in open water. The strategy of eukaryotic sensory integration, sensory processing and the speed and mechanics of tactic responses is fundamentally different from that found in prokaryotes.[39][5]
Both single-celled and multi-cellular eukaryotic phototactic organisms have a fixed shape, are polarized, swim in a spiral and use cilia for swimming and phototactic steering. Signalling can happen via direct light-triggered ion currents, adenylyl cyclases or trimeric G-proteins. The photoreceptors used can also be very different (see below). However, signalling in all cases eventually modifies the beating activity of cilia.[5] The mechanics of phototactic orientation is analogous in all eukaryotes. A photosensor with a restricted view angle rotates to scan the space and signals periodically to the cilia to alter their beating, which will change the direction of the helical swimming trajectory. Three-dimensional phototaxis can be found in five out of the six eukaryotic major groups (opisthokonts, Amoebozoa, plants, chromalveolates, excavates, rhizaria).[5]
Pelagic phototaxis is present in green algae – it is not present in glaucophyte algae or red algae.[5] Green algae have a "stigma" located in the outermost portion of the chloroplast, directly underneath the two chloroplast membranes. The stigma is made of tens to several hundreds of lipid globules, which often form hexagonal arrays and can be arranged in one or more rows. The lipid globules contain a complex mixture of carotenoid pigments, which provide the screening function and the orange-red colour,[40] as well as proteins that stabilize the globules.[41] The stigma is located laterally, in a fixed plane relative to the cilia, but not directly adjacent to the basal bodies.[42][43] The fixed position is ensured by the attachment of the chloroplast to one of the ciliary roots.[44] The pigmented stigma is not to be confused with the photoreceptor. The stigma only provides directional shading for the adjacent membrane-inserted photoreceptors (the term "eyespot" is therefore misleading). Stigmata can also reflect and focus light like a concave mirror, thereby enhancing sensitivity.[5]
In the best-studied green alga, Chlamydomonas reinhardtii, phototaxis is mediated by a rhodopsin pigment, as first demonstrated by the restoration of normal photobehaviour in a blind mutant by analogues of the retinal chromophore.[45] Two archaebacterial-type rhodopsins, channelrhodopsin-1 and -2,[46][47] were identified as phototaxis receptors in Chlamydomonas.[48] Both proteins have an N-terminal 7-transmembrane portion, similar to archaebacterial rhodopsins, followed by an approximately 400 residue C-terminal membrane-associated portion. CSRA and CSRB act as light-gated cation channels and trigger depolarizing photocurrents.[48][49] CSRA was shown to localize to the stigma region using immunofluorescence analysis (Suzuki et al. 2003). Individual RNAi depletion of both CSRA and CSRB modified the light-induced currents and revealed that CSRA mediates a fast, high-saturating current while CSRB a slow, low-saturating one. Both currents are able to trigger photophobic responses and can have a role in phototaxis,[50][49] although the exact contribution of the two receptors is not yet clear.[5]
As in all bikonts (plants, chromalveolates, excavates, rhizaria), green algae have two cilia, which are not identical. The anterior cilium is always younger than the posterior one.[51][52] In every cell cycle, one daughter cell receives the anterior cilium and transforms it into a posterior one. The other daughter inherits the posterior, mature cilium. Both daughters then grow a new anterior cilium.[5]
As all other ciliary swimmers, green algae always swim in a spiral. The handedness of the spiral is robust and is guaranteed by the chirality of the cilia. The two cilia of green algae have different beat patterns and functions. In Chlamydomonas, the phototransduction cascade alters the stroke pattern and beating speed of the two cilia differentially in a complex pattern.[53][54] This results in the reorientation of the helical swimming trajectory as long as the helical swimming axis is not aligned with the light vector.[5]
Phototaxis in invertebrates
Jellyfish
Positive and negative phototaxis can be found in several species of jellyfish such as those from the genus Polyorchis. Jellyfish use ocelli to detect the presence and absence of light, which is then translated into anti-predatory behaviour in the case of a shadow being cast over the ocelli, or feeding behaviour in the case of the presence of light.[55] Many tropical jellyfish have a symbiotic relationship with photosynthetic zooxanthellae that they harbor within their cells.[56] The zooxanthellae nourish the jellyfish, while the jellyfish protects them, and moves them toward light sources such as the sun to maximize their light-exposure for efficient photosynthesis. In a shadow, the jellyfish can either remain still, or quickly move away in bursts to avoid predation and also re-adjust toward a new light source.[57]
This motor response to light and absence of light is facilitated by a chemical response from the ocelli, which results in a motor response causing the organism to swim toward a light source.[57]
Marine ragworm
Phototaxis has been well studied in the marine ragworm Platynereis dumerilii. Both Platynereis dumerilii trochophore and its metatrochophore larvae are positively phototactic. Phototaxis is mediated by simple eyespots that consists of a pigment cell and a photoreceptor cell. The photoreceptor cell synapses directly onto ciliated cells, which are used for swimming. The eyespots do not give spatial resolution, therefore the larvae are rotating to scan their environment for the direction where the light is coming from.[58]
Platynereis dumerilii larvae (nectochaete) can switch between positive and negative phototaxis. Phototaxis there is mediated by two pairs of more complex pigment cup eyes. These eyes contain more photoreceptor cells that are shaded by pigment cells forming a cup. The photoreceptor cells do not synapse directly onto ciliated cells or muscle cells but onto inter-neurons of a processing center. This way the information of all four eye cups can be compared and a low-resolution image of four pixels can be created telling the larvae where the light is coming from. This way the larva does not need to scan its environment by rotating.[59] This is an adaption for living on the bottom of the sea the lifestyle of the larva while scanning rotation is more suited for living in the open water column, the lifestyle of the trochophore larva. Phototaxis in the Platynereis dumerilii larva has a broad spectral range which is at least covered by three opsins that are expressed by the cup eyes:[60] Two rhabdomeric opsins[61] and a Go-opsin.[60]
File:PlatynereisDumeriliiFemaleEpitoke.tif
However, not every behavior that looks like phototaxis is phototaxis: Platynereis dumerilii nechtochate and metatrochophore larvae swim up first when they are stimulated with UV-light from above. But after a while, they change the direction and avoid the UV-light by swimming down. This looks like a change from positive to negative phototaxis (see video left), but the larvae also swim down if UV-light comes non-directionally from the side. And so they do not swim to or away from the light, but swim down,[62] this means to the center of gravity. Thus this is a UV-induced positive gravitaxis. Positive phototaxis (swimming to the light from the surface) and positive gravitaxis (swimming to the center of gravity) are induced by different ranges of wavelengths and cancel out each other at a certain ratio of wavelengths.[62] Since the wavelengths compositions change in water with depth: Short (UV, violet) and long (red) wavelengths are lost first,[60] phototaxis and gravitaxis form a ratio-chromatic depth gauge, which allows the larvae to determine their depth by the color of the surrounding water. This has the advantage over a brightness based depth gauge that the color stays almost constant independent of the time of the day or whether it is cloudy.[63][64]
In the diagram on the right, the larvae start swimming upwards when UV-light switched on (marked by the violet square). But later, they are swimming downward. The larval tracks are color coded: Red for upward and blue for downward swimming larvae. The video runs at double speed.[62]
-
Phototaxis of Platynereis dumerilii larvae: Some larvae show positive phototaxis by swimming towards the light. Other larvae show negative phototaxis by swimming away from the light. First, the light comes from left and then from the right side. When the light direction is switched the larvae turn. The side where the light is coming from is indicated by a white bar. The larvae display mixed phototaxis, some negatively phototactic larvae are tracked. The scale bar represents 2 mm.[59]
-
Phototaxis of Platynereis dumerilii larvae: The larvae turn when the light coming from the left is switched on. While the larvae turn they bend their body with their longitudinal muscles. The larvae show two dots on the head, which are the shading pigment of their adult cup eyes that mediate phototaxis. The direction where the light is coming from is indicated by white bars.[59]
Insects

Positive phototaxis can be found in many flying insects such as moths, grasshoppers, and flies. Drosophila melanogaster has been studied extensively for its innate positive phototactic response to light sources, using controlled experiments to help understand the connection between airborne locomotion toward a light source.[65] This innate response is common among insects that fly primarily during the night utilizing transverse orientation vis-à-vis the light of the moon for orientation.[66] Artificial lighting in cities and populated areas results in a more pronounced positive response compared to that with the distant light of the moon, resulting in the organism repeatedly responding to this new supernormal stimulus and innately flying toward it.
Evidence for the innate response of positive phototaxis in Drosophila melanogaster was carried out by altering the wings of several individual specimens, both physically (via removal) and genetically (via mutation). In both cases there was a noticeable lack of positive phototaxis, demonstrating that flying toward light sources is an innate response to the organisms' photoreceptors receiving a positive response.[65]
Negative phototaxis can be observed in larval drosophila melanogaster within the first three developmental instar stages, despite adult insects displaying positive phototaxis.[67] This behaviour is common among other species of insects which possess a flightless larval and adult stage in their life cycles, only switching to positive phototaxis when searching for pupation sites. Tenebrio molitor by comparison is one species which carries its negative phototaxis into adulthood.[67]
Relation to magnetic fields
Under experimental conditions, organisms that use positive phototaxis have also shown a correlation with light and magnetic fields. Under homogeneous light conditions with a shifting magnetic field, Drosophila melanogaster larvae reorient themselves toward predicted directions of greater or lesser light intensities as expected by a rotating magnetic field. In complete darkness, the larvae orient randomly without any notable preference.[67] This suggests the larvae can observe a visible pattern in combination with light.
Light based vertical movement
A depth can also be selected based on light levels: The brightness decreases with depth, but depends on the weather (e.g. whether it is sunny or cloudy) and the time of the day. Also the color depends on the water depth and dissolved and suspended matter.[63][64] The only consistent factor is that at a given place, deeper water is darker.
In water, light attenuates differently for each wavelength. The UV, violet (> 420 nm), and red (< 500 nm) wavelengths disappear before blue light (470 nm), which penetrates clear water the deepest.[68][60] The wavelength composition is constant for each depth and is almost independent of time of the day and the weather. To gauge depth, an animal would need two photopigments sensitive to different wavelengths to compare different ranges of the spectrum.[63][64] Such pigments may be expressed in different structures.
Such different structures are found in the polychaete Torrea candida. Its eyes have a main and two accessory retinae. The accessory retinae sense UV-light (λmax = 400 nm) and the main retina senses blue-green light (λmax = 560 nm). If the light sensed from all retinae is compared, the depth can be estimated, and so for Torrea candida such a ratio-chromatic depth gauge has been proposed.[69]
A ratio chromatic depth gauge has been found in larvae of the polychaete Platynereis dumerilii.[62] The larvae have two structures: The rhabdomeric photoreceptor cells of the eyes[70] and in the deep brain the ciliary photoreceptor cells. The ciliary photoreceptor cells express a ciliary opsin,[71] which is a photopigment maximally sensitive to UV-light (λmax = 383 nm).[72] Thus, the ciliary photoreceptor cells react on UV-light and make the larvae swimming down gravitactically. The gravitaxis here is countered by phototaxis, which makes the larvae swimming up to the light coming from the surface.[60] Phototaxis is mediated by the rhabdomeric eyes.[59][62] The eyes express at least three opsins (at least in the older larvae),[61] and one of them is maximally sensitive to cyan light (λmax = 483 nm) so that the eyes cover a broad wavelength range with phototaxis.[60] When phototaxis and gravitaxis have leveled out, the larvae have found their preferred depth.[62]
See also
- Photokinesis
- Phototropism (more relevant to plants and fungi)
References
- ↑ Martin, E.A., ed. (1983), Macmillan Dictionary of Life Sciences (2nd ed.), London: Macmillan Press, p. 362, ISBN 978-0-333-34867-3
- ↑ 2.0 2.1 Menzel, Randolf (1979), "Spectral Sensitivity and Color Vision in Invertebrates", in H. Autrum, Comparative Physiology and Evolution of Vision in Invertebrates- A: Invertebrate Photoreceptors, Handbook of Sensory Physiology, VII/6A, New York: Springer-Verlag, pp. 503–580. See section D: Wavelength–Specific Behavior and Color Vision, ISBN 978-3-540-08837-0
- ↑ Scharf, Birgit; Wolff, Elmar K. (1994). "Phototactic behaviour of the archaebacterial Natronobacterium pharaonis". FEBS Letters 340 (1–2): 114–116. doi:10.1016/0014-5793(94)80183-5. PMID 8119392.
- ↑ Armitage, Judith P.; Hellingwerf, Klaas J. (2003). "Light-induced behavioral responses (;phototaxis') in prokaryotes". Photosynthesis Research 76 (1–3): 145–155. doi:10.1023/A:1024974111818. PMID 16228574.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 Jékely, Gáspár (2009). "Evolution of phototaxis". Philosophical Transactions of the Royal Society B: Biological Sciences 364 (1531): 2795–2808. doi:10.1098/rstb.2009.0072. PMID 19720645. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Luecke, H.; Schobert, B.; Lanyi, J. K.; Spudich, E. N.; Spudich, J. L. (2001). "Crystal Structure of Sensory Rhodopsin II at 2.4 Angstroms: Insights into Color Tuning and Transducer Interaction". Science 293 (5534): 1499–1503. doi:10.1126/science.1062977. PMID 11452084. Bibcode: 2001Sci...293.1499L.
- ↑ Spudich, John L. (2006). "The multitalented microbial sensory rhodopsins". Trends in Microbiology 14 (11): 480–487. doi:10.1016/j.tim.2006.09.005. PMID 17005405.
- ↑ Yan, B.; Takahashi, T.; Johnson, R.; Derguini, F.; Nakanishi, K.; Spudich, J.L. (1990). "All-trans/13-cis isomerization of retinal is required for phototaxis signaling by sensory rhodopsins in Halobacterium halobium". Biophysical Journal 57 (4): 807–814. doi:10.1016/S0006-3495(90)82600-X. PMID 2344465. Bibcode: 1990BpJ....57..807Y.
- ↑ Gordeliy, Valentin I.; Labahn, Jörg; Moukhametzianov, Rouslan; Efremov, Rouslan; Granzin, Joachim; Schlesinger, Ramona; Büldt, Georg; Savopol, Tudor et al. (2002). "Molecular basis of transmembrane signalling by sensory rhodopsin II–transducer complex". Nature 419 (6906): 484–487. doi:10.1038/nature01109. PMID 12368857. Bibcode: 2002Natur.419..484G.
- ↑ Sasaki, Jun; Spudich, John L. (2008). "Signal Transfer in Haloarchaeal Sensory Rhodopsin Transducer Complexes". Photochemistry and Photobiology 84 (4): 863–868. doi:10.1111/j.1751-1097.2008.00314.x. PMID 18346091.
- ↑ Rudolph, J.; Oesterhelt, D. (1995). "Chemotaxis and phototaxis require a CheA histidine kinase in the archaeon Halobacterium salinarium". The EMBO Journal 14 (4): 667–673. doi:10.1002/j.1460-2075.1995.tb07045.x. PMID 7882970.
- ↑ McCain, D. A.; Amici, L. A.; Spudich, J. L. (1987). "Kinetically resolved states of the Halobacterium halobium flagellar motor switch and modulation of the switch by sensory rhodopsin I". Journal of Bacteriology 169 (10): 4750–4758. doi:10.1128/jb.169.10.4750-4758.1987. PMID 3654583.
- ↑ Nultsch, Wilhelm; Schuchart, Hartwig; Höhl, Marga (1979). "Investigations on the phototactic orientation of Anabaena variabilis". Archives of Microbiology 122: 85–91. doi:10.1007/BF00408050.
- ↑ Choi, Jong-Soon; Chung, Young-Ho; Moon, Yoon-Jung; Kim, Changhoon; Watanabe, Masakatsu; Song, Pill-Soon; Joe, Cheol-O; Bogorad, Lawrence et al. (1999). "Photomovement of the Gliding Cyanobacterium Synechocystis sp. PCC 6803". Photochemistry and Photobiology 70 (1): 95–102. doi:10.1111/j.1751-1097.1999.tb01954.x. PMID 10420848.
- ↑ Bhoo, Seong-Hee; Davis, Seth J.; Walker, Joseph; Karniol, Baruch; Vierstra, Richard D. (2001). "Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore". Nature 414 (6865): 776–779. doi:10.1038/414776a. PMID 11742406. Bibcode: 2001Natur.414..776B.
- ↑ Zhulin, I.B. (2000) "A novel phototaxis receptor hidden in the cyanobacterial genome". Journal of molecular microbiology and biotechnology, 2(4): 491–494.
- ↑ 17.0 17.1 Bhaya, Devaki (2004). "Light matters: Phototaxis and signal transduction in unicellular cyanobacteria". Molecular Microbiology 53 (3): 745–754. doi:10.1111/j.1365-2958.2004.04160.x. PMID 15255889.
- ↑ Yoshihara, Shizue; Ikeuchi, Masahiko (2004). "Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC 6803". Photochemical & Photobiological Sciences 3 (6): 512–518. doi:10.1039/b402320j. PMID 15170479.
- ↑ Yoshihara, Shizue; Suzuki, Fumiko; Fujita, Hironori; Geng, Xiao Xing; Ikeuchi, Masahiko (2000). "Novel Putative Photoreceptor and Regulatory Genes Required for the Positive Phototactic Movement of the Unicellular Motile Cyanobacterium Synechocystis sp. PCC 6803". Plant and Cell Physiology 41 (12): 1299–1304. doi:10.1093/pcp/pce010. PMID 11134414.
- ↑ Gestwicki, Jason E.; Lamanna, Allison C.; Harshey, Rasika M.; McCarter, Linda L.; Kiessling, Laura L.; Adler, Julius (2000). "Evolutionary Conservation of Methyl-Accepting Chemotaxis Protein Location in Bacteria and Archaea". Journal of Bacteriology 182 (22): 6499–6502. doi:10.1128/JB.182.22.6499-6502.2000. PMID 11053396.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 21.6 Wilde, Annegret; Mullineaux, Conrad W. (2017). "Light-controlled motility in prokaryotes and the problem of directional light perception". FEMS Microbiology Reviews 41 (6): 900–922. doi:10.1093/femsre/fux045. PMID 29077840. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 22.0 22.1 22.2 22.3 Häder, D. P. (1987). "Photosensory behavior in procaryotes". Microbiological Reviews 51 (1): 1–21. doi:10.1128/mr.51.1.1-21.1987. PMID 3104747.
- ↑ 23.0 23.1 Armitage, Judith P.; Hellingwerf, Klaas J. (2003). "Light-induced behavioral responses (;phototaxis') in prokaryotes". Photosynthesis Research 76 (1–3): 145–155. doi:10.1023/A:1024974111818. PMID 16228574.
- ↑ Raymond, J.; Zhaxybayeva, O.; Gogarten, J. P.; Gerdes, S. Y.; Blankenship, R. E. (2002). "Whole-Genome Analysis of Photosynthetic Prokaryotes". Science 298 (5598): 1616–1620. doi:10.1126/science.1075558. PMID 12446909. Bibcode: 2002Sci...298.1616R.
- ↑ Rastogi, Rajesh Prasad; Sinha, Rajeshwar P.; Moh, Sang Hyun; Lee, Taek Kyun; Kottuparambil, Sreejith; Kim, Youn-Jung; Rhee, Jae-Sung; Choi, Eun-Mi et al. (2014). "Ultraviolet radiation and cyanobacteria". Journal of Photochemistry and Photobiology B: Biology 141: 154–169. doi:10.1016/j.jphotobiol.2014.09.020. PMID 25463663.
- ↑ Yutthanasirikul, Rayakorn; Nagano, Takanori; Jimbo, Haruhiko; Hihara, Yukako; Kanamori, Takashi; Ueda, Takuya; Haruyama, Takamitsu; Konno, Hiroki et al. (2016). "Oxidation of a Cysteine Residue in Elongation Factor EF-Tu Reversibly Inhibits Translation in the Cyanobacterium Synechocystis sp. PCC 6803". Journal of Biological Chemistry 291 (11): 5860–5870. doi:10.1074/jbc.M115.706424. PMID 26786107.
- ↑ Purcell, Erin B.; Crosson, Sean (2008). "Photoregulation in prokaryotes". Current Opinion in Microbiology 11 (2): 168–178. doi:10.1016/j.mib.2008.02.014. PMID 18400553.
- ↑ Bonomi, Hernán R.; Toum, Laila; Sycz, Gabriela; Sieira, Rodrigo; Toscani, Andrés M.; Gudesblat, Gustavo E.; Leskow, Federico C.; Goldbaum, Fernando A. et al. (2016). "Xanthomonas campestris attenuates virulence by sensing light through a bacteriophytochrome photoreceptor". EMBO Reports 17 (11): 1565–1577. doi:10.15252/embr.201541691. PMID 27621284.
- ↑ Damerval, T.; Guglielmi, G.; Houmard, J.; De Marsac, N. T. (1991). "Hormogonium Differentiation in the Cyanobacterium Calothrix: A Photoregulated Developmental Process". The Plant Cell 3 (2): 191–201. doi:10.1105/tpc.3.2.191. PMID 12324595.
- ↑ Richardson, Laurie L.; Castenholz, Richard W. (1987). "Diel Vertical Movements of the Cyanobacterium Oscillatoria terebriformis in a Sulfide-Rich Hot Spring Microbial Mat". Applied and Environmental Microbiology 53 (9): 2142–2150. doi:10.1128/aem.53.9.2142-2150.1987. PMID 16347435. Bibcode: 1987ApEnM..53.2142R.
- ↑ Stal, Lucas J. (1995). "Physiological ecology of cyanobacteria in microbial mats and other communities". New Phytologist 131 (1): 1–32. doi:10.1111/j.1469-8137.1995.tb03051.x. PMID 33863161. https://pure.uva.nl/ws/files/2891064/948_10981y.pdf.
- ↑ Yang, H.; Inokuchi, H.; Adler, J. (1995). "Phototaxis away from blue light by an Escherichia coli mutant accumulating protoporphyrin IX". Proceedings of the National Academy of Sciences 92 (16): 7332–7336. doi:10.1073/pnas.92.16.7332. PMID 7638191. Bibcode: 1995PNAS...92.7332Y.
- ↑ Engelmann, Th. W. (1883). "Bacterium photometricum". Pflügers Archiv für die gesamte Physiologie des Menschen und der Tiere 30: 95–124. doi:10.1007/BF01674325. https://zenodo.org/record/1761254.
- ↑ Wadhams, George H.; Armitage, Judith P. (2004). "Making sense of it all: Bacterial chemotaxis". Nature Reviews Molecular Cell Biology 5 (12): 1024–1037. doi:10.1038/nrm1524. PMID 15573139.
- ↑ Kreimer, Georg (2009). "The green algal eyespot apparatus: A primordial visual system and more?". Current Genetics 55 (1): 19–43. doi:10.1007/s00294-008-0224-8. PMID 19107486.
- ↑ Ragatz, Lisa; Jiang, Ze-Yu; Bauer, Carl; Gest, Howard (1994). "Phototactic purple bacteria". Nature 370 (6485): 104. doi:10.1038/370104a0. Bibcode: 1994Natur.370..104R.
- ↑ Ueki, Noriko; Matsunaga, Shigeru; Inouye, Isao; Hallmann, Armin (2010). "How 5000 independent rowers coordinate their strokes in order to row into the sunlight: Phototaxis in the multicellular green alga Volvox". BMC Biology 8: 103. doi:10.1186/1741-7007-8-103. PMID 20663212.
- ↑ Clark, M.A., Choi, J. and Douglas, M. (2018) Characteristics of Protists Biology 2e. OpenStax. ISBN 9781947172951. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License
- ↑ Häder, D. -P; Lebert, M. (19 June 2001). Photomovement. Elsevier. ISBN 978-0-08-053886-0. https://books.google.com/books?id=2nevsljDiCYC&pg=PP1.
- ↑ Grung, Merete; Kreimer, Georg; Calenberg, Michael; Melkonian, Michael; Liaaen-Jensen, Synnøve (1994). "Carotenoids in the eyespot apparatus of the flagellate green alga Spermatozopsis similis: Adaptation to the retinal-based photoreceptor". Planta 193. doi:10.1007/BF00191604.
- ↑ Renninger, S.; Backendorf, E.; Kreimer, G. (2001). "Subfractionation of eyespot apparatuses from the green alga Spermatozopsis similis: Isolation and characterization of eyespot globules". Planta 213 (1): 51–63. doi:10.1007/s004250000473. PMID 11523656.
- ↑ Arnott, Howard J.; Brown, R. Malcolm (1967). "Ultrastructure of the Eyespot and its Possible Significance in Phototaxis of Tetracystis excentrica*†". The Journal of Protozoology 14 (4): 529–539. doi:10.1111/j.1550-7408.1967.tb02038.x.
- ↑ Melkonian, M.; Robenek, H. (1979). "The eyespot of the flagellate Tetraselmis cordiformis stein (Chlorophyceae): Structural spezialization of the outer chloroplast membrane and its possible significance in phototaxis of green algae". Protoplasma 100 (2): 183–197. doi:10.1007/BF01283929.
- ↑ Melkonian, Michael (1978). "Structure and significance of cruciate flagellar root systems in green algae: Comparative investigations in species of Chlorosarcinopsis (Chlorosarcinales)". Plant Systematics and Evolution 130 (3–4): 265–292. doi:10.1007/BF00982810.
- ↑ Foster, Kenneth W.; Saranak, Jureepan; Patel, Nayana; Zarilli, Gerald; Okabe, Masami; Kline, Toni; Nakanishi, Koji (1984). "A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas". Nature 311 (5988): 756–759. doi:10.1038/311756a0. PMID 6493336. Bibcode: 1984Natur.311..756F.
- ↑ Nagel, G.; Ollig, D.; Fuhrmann, M.; Kateriya, S.; Musti, A. M.; Bamberg, E.; Hegemann, P. (2002). "Channelrhodopsin-1: A Light-Gated Proton Channel in Green Algae". Science 296 (5577): 2395–2398. doi:10.1126/science.1072068. PMID 12089443. Bibcode: 2002Sci...296.2395N.
- ↑ Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P. et al. (2003). "Channelrhodopsin-2, a directly light-gated cation-selective membrane channel". Proceedings of the National Academy of Sciences 100 (24): 13940–13945. doi:10.1073/pnas.1936192100. PMID 14615590. Bibcode: 2003PNAS..10013940N.
- ↑ 48.0 48.1 Sineshchekov, O. A.; Jung, K.-H.; Spudich, J. L. (2002). "Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii". Proceedings of the National Academy of Sciences 99 (13): 8689–8694. doi:10.1073/pnas.122243399. PMID 12060707.
- ↑ 49.0 49.1 Berthold, Peter; Tsunoda, Satoshi P.; Ernst, Oliver P.; Mages, Wolfgang; Gradmann, Dietrich; Hegemann, Peter (2008). "Channelrhodopsin-1 Initiates Phototaxis and Photophobic Responses in Chlamydomonas by Immediate Light-Induced Depolarization". The Plant Cell 20 (6): 1665–1677. doi:10.1105/tpc.108.057919. PMID 18552201.
- ↑ Govorunova, Elena G.; Jung, Kwang-Hwan; Sineshchekov, Oleg A.; Spudich, John L. (2004). "Chlamydomonas Sensory Rhodopsins a and B: Cellular Content and Role in Photophobic Responses". Biophysical Journal 86 (4): 2342–2349. doi:10.1016/S0006-3495(04)74291-5. PMID 15041672. Bibcode: 2004BpJ....86.2342G.
- ↑ Cavalier-Smith, T. (2002). "The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa". International Journal of Systematic and Evolutionary Microbiology 52 (2): 297–354. doi:10.1099/00207713-52-2-297. PMID 11931142.
- ↑ Cavalier-Smith, Thomas (2009). "Megaphylogeny, Cell Body Plans, Adaptive Zones: Causes and Timing of Eukaryote Basal Radiations". Journal of Eukaryotic Microbiology 56 (1): 26–33. doi:10.1111/j.1550-7408.2008.00373.x. PMID 19340985.
- ↑ Josef, Keith; Saranak, Jureepan; Foster, Kenneth W. (2005). "Ciliary behavior of a negatively phototactic Chlamydomonas reinhardtii". Cell Motility and the Cytoskeleton 61 (2): 97–111. doi:10.1002/cm.20069. PMID 15849714.
- ↑ Josef, Keith; Saranak, Jureepan; Foster, Kenneth W. (2006). "Linear systems analysis of the ciliary steering behavior associated with negative-phototaxis in Chlamydomonas reinhardtii". Cell Motility and the Cytoskeleton 63 (12): 758–777. doi:10.1002/cm.20158. PMID 16986140.
- ↑ Katsuki, Takeo; Greenspan, Ralph J. (July 2013). "Jellyfish nervous systems". Current Biology 23 (14): R592–R594. doi:10.1016/j.cub.2013.03.057. ISSN 0960-9822. PMID 23885868.
- ↑ E., Ruppert, Edward (2004). Invertebrate zoology: a functional evolutionary approach. Barnes, Robert D.,, Fox, Richard S. (Seventh ed.). Delhi, India. ISBN 978-81-315-0104-7. OCLC 970002268.
- ↑ 57.0 57.1 Anderson, P.; Mackie, G. (1977-07-08). "Electrically coupled, photosensitive neurons control swimming in a jellyfish". Science 197 (4299): 186–188. doi:10.1126/science.17918. ISSN 0036-8075. PMID 17918. Bibcode: 1977Sci...197..186A.
- ↑ Jékely, Gáspár; Colombelli, Julien; Hausen, Harald; Guy, Keren; Stelzer, Ernst; Nédélec, François; Arendt, Detlev (20 November 2008). "Mechanism of phototaxis in marine zooplankton". Nature 456 (7220): 395–399. doi:10.1038/nature07590. PMID 19020621. Bibcode: 2008Natur.456..395J.
- ↑ 59.0 59.1 59.2 59.3 Randel, Nadine; Asadulina, Albina; Bezares-Calderón, Luis A; Verasztó, Csaba; Williams, Elizabeth A; Conzelmann, Markus; Shahidi, Réza; Jékely, Gáspár (27 May 2014). "Neuronal connectome of a sensory-motor circuit for visual navigation". eLife 3. doi:10.7554/eLife.02730. PMID 24867217.
- ↑ 60.0 60.1 60.2 60.3 60.4 60.5 Gühmann, Martin; Jia, Huiyong; Randel, Nadine; Verasztó, Csaba; Bezares-Calderón, Luis A.; Michiels, Nico K.; Yokoyama, Shozo; Jékely, Gáspár (August 2015). "Spectral Tuning of Phototaxis by a Go-Opsin in the Rhabdomeric Eyes of Platynereis". Current Biology 25 (17): 2265–2271. doi:10.1016/j.cub.2015.07.017. PMID 26255845.
- ↑ 61.0 61.1 Randel, N.; Bezares-Calderon, L. A.; Gühmann, M.; Shahidi, R.; Jekely, G. (10 May 2013). "Expression Dynamics and Protein Localization of Rhabdomeric Opsins in Platynereis Larvae". Integrative and Comparative Biology 53 (1): 7–16. doi:10.1093/icb/ict046. PMID 23667045.
- ↑ 62.0 62.1 62.2 62.3 62.4 62.5 Verasztó, Csaba; Gühmann, Martin; Jia, Huiyong; Rajan, Vinoth Babu Veedin; Bezares-Calderón, Luis A.; Piñeiro-Lopez, Cristina; Randel, Nadine; Shahidi, Réza et al. (29 May 2018). "Ciliary and rhabdomeric photoreceptor-cell circuits form a spectral depth gauge in marine zooplankton". eLife 7. doi:10.7554/eLife.36440. PMID 29809157.
- ↑ 63.0 63.1 63.2 Nilsson, Dan-Eric (31 August 2009). "The evolution of eyes and visually guided behavior". Philosophical Transactions of the Royal Society B: Biological Sciences 364 (1531): 2833–2847. doi:10.1098/rstb.2009.0083. PMID 19720648.
- ↑ 64.0 64.1 64.2 Nilsson, Dan-Eric (12 April 2013). "Eye evolution and its functional basis". Visual Neuroscience 30 (1–2): 5–20. doi:10.1017/S0952523813000035. PMID 23578808.
- ↑ 65.0 65.1 Gorostiza, E. Axel; Colomb, Julien; Brembs, Bjoern (2015-08-03). "A decision underlies phototaxis in an insect.". Open Biology 6 (12). doi:10.1098/rsob.160229. PMID 28003472.
- ↑ Reynolds, Andy M.; Reynolds, Don R.; Sane, Sanjay P.; Hu, Gao; Chapman, Jason W. (2016-08-15). "Orientation in high-flying migrant insects in relation to flows: mechanisms and strategies". Philosophical Transactions of the Royal Society B: Biological Sciences 371 (1704). doi:10.1098/rstb.2015.0392. ISSN 0962-8436. PMID 27528782.
- ↑ 67.0 67.1 67.2 Riveros, A.J.; Srygley, R.B. (2010), Magnetic Compasses in Insects, Elsevier, pp. 305–313, doi:10.1016/b978-0-08-045337-8.00075-9, ISBN 978-0-08-045337-8
- ↑ Lythgoe, John N. (1988). "Light and Vision in the Aquatic Environment" (in en). Sensory Biology of Aquatic Animals. pp. 57–82. doi:10.1007/978-1-4612-3714-3_3. ISBN 978-1-4612-8317-1.
- ↑ Wald, George; Rayport, Stephen (24 June 1977). "Vision in Annelid Worms". Science 196 (4297): 1434–1439. doi:10.1126/science.196.4297.1434. PMID 17776921. Bibcode: 1977Sci...196.1434W.
- ↑ Rhode, Birgit (April 1992). "Development and differentiation of the eye in Platynereis dumerilii (Annelida, Polychaeta)". Journal of Morphology 212 (1): 71–85. doi:10.1002/jmor.1052120108. PMID 29865584.
- ↑ Arendt, D.; Tessmar-Raible, K.; Snyman, H.; Dorresteijn, A.W.; Wittbrodt, J. (29 October 2004). "Ciliary Photoreceptors with a Vertebrate-Type Opsin in an Invertebrate Brain". Science 306 (5697): 869–871. doi:10.1126/science.1099955. PMID 15514158. Bibcode: 2004Sci...306..869A.
- ↑ Tsukamoto, Hisao; Chen, I-Shan; Kubo, Yoshihiro; Furutani, Yuji (4 August 2017). "A ciliary opsin in the brain of a marine annelid zooplankton is ultraviolet-sensitive, and the sensitivity is tuned by a single amino acid residue" (in en). Journal of Biological Chemistry 292 (31): 12971–12980. doi:10.1074/jbc.M117.793539. ISSN 0021-9258. PMID 28623234.
Further reading
- Madigan, Michael T.; Martinko, John M. (2006). Brock Biology of Microorganisms (11th ed.). Upper Saddle River, N.J.: Pearson/Prentice Hall. ISBN 978-0-13-144329-7.
- Jékely, G. (31 August 2009). "Evolution of phototaxis". Philosophical Transactions of the Royal Society B: Biological Sciences 364 (1531): 2795–2808. doi:10.1098/rstb.2009.0072. PMID 19720645.
- Randel, Nadine; Jékely, Gáspár (23 November 2015). "Phototaxis and the origin of visual eyes". Philosophical Transactions of the Royal Society B: Biological Sciences 371 (1685). doi:10.1098/rstb.2015.0042. PMID 26598725.
 |
