Biology:Neuraminidase inhibitor
Neuraminidase inhibitors (NAIs) are a class of drugs which block the neuraminidase enzyme. They are a commonly used antiviral drug type against influenza. Viral neuraminidases are essential for influenza reproduction, facilitating viral budding from the host cell. Oseltamivir (Tamiflu), zanamivir (Relenza), laninamivir (Inavir), and peramivir belong to this class. Unlike the M2 inhibitors, which work only against the influenza A virus, NAIs act against both influenza A and influenza B.[1][2][3][4] The NAIs oseltamivir and zanamivir were approved in the US and Europe for treatment and prevention of influenza A and B. Peramivir acts by strongly binding to the neuraminidase of the influenza viruses and inhibits activation of neuraminidase much longer than oseltamivir or zanamivir.[5] However, laninamivir in the cells is slowly released into the respiratory tract, resulting in long-lasting anti-influenza virus activity. Thus the mechanism of the long-lasting activity of laninamivir is basically different from that of peramivir.[6]
The efficacy was highly debated in recent years.[7][8][9] However, after the pandemic caused by H1N1 in 2009, the effectiveness of early treatment with neuraminidase inhibitors in reducing serious cases and deaths was reported in various countries.[10][11][12][13]
In countries where influenza-like illness is treated using NAIs on a national level, statistical reports show a low fatality record for symptomatic illness because of the universal implementation of early treatment using this class of drugs.[14] Although oseltamivir is widely used in these countries, there have been no outbreaks caused by oseltamivir-resistant viruses and also no serious illness caused by oseltamivir-resistant viruses has ever been reported.[14] The United States Centers for Disease Control and Prevention continues to recommend the use of oseltamavir treatment for people at high risk for complications and the elderly and those at lower risk who present within 48 hours of first symptoms of infection.[15]
Common side effects include nausea and vomiting. The abnormal behaviors of children after taking oseltamivir that have been reported may be an extension of delirium or hallucinations caused by influenza.[14] It occurs in the early stages of the illness, such as within 48 hours after onset of the illness. Therefore, children with influenza are advised to be observed by their parents until 48 hours after the onset of the influenza illness, regardless of whether the child is treated with NAIs.[14]
Specific neuraminidase inhibitors
- Laninamivir
- Oseltamivir (Tamiflu)
- Peramivir (Rapivab)
- Zanamivir (Relenza)
Structures of the viral neuraminidase inhibitors in use
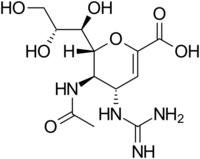
|
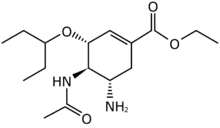
|
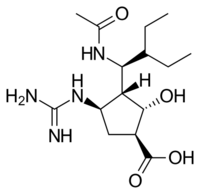
|
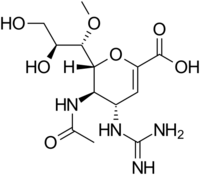
|
| Zanamivir | Oseltamivir | Peramivir | Laninamivir |
Natural products
- Cyanidin-3-sambubioside (extracted from black elderberry)[16]
- Coptisine[citation needed]
- Berberine[17]
See also
References
- ↑ "Structural studies of the resistance of influenza virus neuramindase to inhibitors". Journal of Medicinal Chemistry 45 (11): 2207–2212. May 2002. doi:10.1021/jm010528u. PMID 12014958.
- ↑ "Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors". Virus Research 103 (1–2): 199–203. July 2004. doi:10.1016/j.virusres.2004.02.034. PMID 15163510.
- ↑ This flash animation shows the mode of action of oseltamivir (Tamiflu) . pharmasquare.org
- ↑ Replication of influenza virus . mvm.ed.ac.uk
- ↑ "Anti-influenza virus activity of peramivir in mice with single intramuscular injection". Antiviral Research 69 (1): 39–45. January 2006. doi:10.1016/j.antiviral.2005.10.002. PMID 16325932.
- ↑ "Clinical pharmacokinetics of laninamivir, a novel long-acting neuraminidase inhibitor, after single and multiple inhaled doses of its prodrug, CS-8958, in healthy male volunteers". Journal of Clinical Pharmacology 50 (11): 1319–1329. November 2010. doi:10.1177/0091270009356297. PMID 20145259.
- ↑ "Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments". BMJ 348: g2545. April 2014. doi:10.1136/bmj.g2545. PMID 24811411.
- ↑ "Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies". Annals of Internal Medicine 156 (7): 512–524. April 2012. doi:10.7326/0003-4819-156-7-201204030-00411. PMID 22371849.
- ↑ "Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments". BMJ 348: g2547. April 2014. doi:10.1136/bmj.g2547. PMID 24811412.
- ↑ "Severe 2009 H1N1 influenza in pregnant and postpartum women in California". The New England Journal of Medicine 362 (1): 27–35. January 2010. doi:10.1056/nejmoa0910444. PMID 20032319.
- ↑ "Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009". The New England Journal of Medicine 361 (20): 1935–1944. November 2009. doi:10.1056/NEJMoa0906695. PMID 19815859.
- ↑ "Critically Ill patients with 2009 influenza A(H1N1) in Mexico". JAMA 302 (17): 1880–1887. November 2009. doi:10.1001/jama.2009.1536. PMID 19822626.
- ↑ "Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection". CMAJ 182 (3): 257–264. February 2010. doi:10.1503/cmaj.091884. PMID 20093297.
- ↑ 14.0 14.1 14.2 14.3 "Widespread use of neuraminidase inhibitors in Japan". Journal of Infection and Chemotherapy 17 (5): 595–601. October 2011. doi:10.1007/s10156-011-0288-0. PMID 21850418.
- ↑ "CDC Online Newsroom - "Have You Heard?" Archive: 2014 - Influenza A Variant Virus". cdc.gov. https://www.cdc.gov/media/haveyouheard/stories/Influenza_antiviral2.html.
- ↑ "Binding of a natural anthocyanin inhibitor to influenza neuraminidase by mass spectrometry". Analytical and Bioanalytical Chemistry 405 (20): 6563–6572. August 2013. doi:10.1007/s00216-013-7068-x. PMID 23748498.
- ↑ "Neuraminidase inhibitory activities of quaternary isoquinoline alkaloids from Corydalis turtschaninovii rhizome". Bioorganic & Medicinal Chemistry 22 (21): 6047–6052. November 2014. doi:10.1016/j.bmc.2014.09.004. PMID 25277281.
External links
 |

