Chemistry:Phenacyl bromide
From HandWiki
Revision as of 11:19, 29 June 2021 by imported>QCDvac (change)
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Bromo-1-phenylethan-1-one | |
| Other names
2-Bromo-1-phenylethanone
2-Bromoacetophenone α-Bromoacetophenone Bromomethyl phenyl ketone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H7BrO | |
| Molar mass | 199.047 g·mol−1 |
| Appearance | Colorless solid |
| Melting point | 50 °C (122 °F; 323 K)[1] |
| Boiling point | 136 °C (277 °F; 409 K) 18 mm Hg[1] |
| Hazards | |
| Main hazards | Toxic(T) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
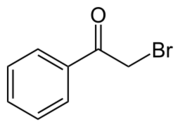
Phenacyl bromide is the organic compound with the formula C6H5C(O)CH2Br. This colourless solid is a powerful lachrymator as well as a useful precursor to other organic compounds.
It is prepared by bromination of acetophenone:[2]
- C6H5C(O)CH3 + Br2 → C6H5C(O)CH2Br + HBr
The compound was first reported in 1871.[3]
References
- ↑ 1.0 1.1 Phenacyl Bromide, TCI America
- ↑ R. M. Cowper and L. H. Davidson. "Phenacyl bromide". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV2P0480.; Collective Volume, 2, pp. 480
- ↑ A. Emmerling and C. Engler (1871). "Ueber einige Abkömmlinge des Acetophenons". Ber. 4 (1): 147–149. doi:10.1002/cber.18710040149. https://zenodo.org/record/1425006.
External links
 |

