Biology:Arctodus
| Arctodus | |
|---|---|

| |
| A. simus from the La Brea Tar Pits | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Family: | Ursidae |
| Subfamily: | Tremarctinae |
| Genus: | †Arctodus Leidy, 1854 |
| Type species | |
| †Arctodus pristinus Leidy, 1854
| |
| Other species | |
| |
| |
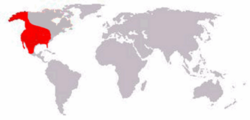
| Arctodus simus range | |
| Synonyms | |
|
synonyms of A. pristinus
synonyms of A. simus
| |
Arctodus is an extinct genus of short-faced bears that inhabited North America during the Pleistocene (about 2.6 Mya until 12,800 years ago). The two recognized species are the lesser short-faced bear (Arctodus pristinus) and the giant short-faced bear (Arctodus simus). Of these species, A. simus was larger, is known from more complete remains, and is considered one of the best-known members of North America's extinct Ice Age megafauna. A. pristinus was largely restricted to the Early Pleistocene of the eastern United States, whereas A. simus had a broader range, with most finds being from the Late Pleistocene of the United States, Mexico and Canada. A. simus evolved from A. pristinus, but both species likely overlapped in the Middle Pleistocene. Both species are relatively rare in the fossil record.
Today considered to be an enormous omnivore, Arctodus simus is believed to be one of the largest known terrestrial carnivorans that has ever existed. Arctodus, like other bears, was highly sexually dimorphic. Adult A. simus ranged between 300 and 950 kilograms (660 and 2,090 lb), with females clustering at ≤500 kg (1,100 lb), and males around 800 kg (1,800 lb). The largest males stood at 1.67 m (5 ft 5.7 in) at the shoulder, and up to 3.4 m (11 ft) tall on their rear legs. Studies suggest that A. simus browsed on C3 vegetation and consumed browsing herbivores such as deer, camelids, and tapir. The species preferred temperate open woodlands, but was adaptable, taking advantage of many habitats and feeding opportunities.
Arctodus belongs to the Tremarctinae subfamily of bears, which are endemic to the Americas. Of these short-faced bears, Arctodus was the most widespread in North America, but was restricted to the Pleistocene. A. pristinus went extinct around 300,000 years ago, with A. simus disappearing about 12,800 years ago in the Late Pleistocene extinctions. The cause behind these extinctions is unclear, but in the case of A. pristinus, was likely due to climate change and competition with other ursids, such as the black bear and Tremarctos floridanus. A. simus likely went extinct due to ecological collapse disrupting the vegetation and prey on which it relied.
Taxonomy

Arctodus was first described by Joseph Leidy in 1854, with finds of A. pristinus from the Ashley Phosphate Beds, South Carolina.[1][2][3] The scientific name of the genus, Arctodus, derives from Greek, and means "bear tooth". The first fossils of A. simus were found in the Potter Creek Cave, Shasta County, California, by J. A. Richardson in 1878, and were initially described as Arctotherium simum by Edward Drinker Cope in 1879.[4][5][6] Historically, all specimens were grouped together under A. pristinus until a revision by Björn Kurtén in 1967.[7] The (lost) holotype and neotype of A. pristinus are both from South Carolina.[8]
In the 19th and early 20th centuries, specimens of Arctodus were occasionally referred to Arctotherium, and vice versa.[6][9][10][11][12][13] Today, however, the genera are not considered to have overlapped, with the closest point of contact being México, with the giant Arctodus simus in Valsequillo, Puebla,[7][14][15] and the smaller Arctotherium wingei in the Yucatán Peninsula.[16] Other early researchers believed Arctodus to be a sister lineage of the agriotheriin Indarctos.[17] Sometimes described as the "American cave bear",[4][18][13] Arctodus should not be mistaken for the similarly large Eurasian cave bear (Ursus spelaeus). As an ursine, the Eurasian cave bear last shared a common ancestor with the tremarctine Arctodus circa 13.4 Mya.[19][20]
Fossils of Arctodus pristinus can be confused with the similarly sized, partially contemporaneous short-faced bear, Tremarctos floridanus.[1] Arctodus has higher crowned and considerably larger teeth than its relative Tremarctos. A. pristinus can be distinguished by broader and taller molars on average, but as they are often worn, differentiation can be difficult.[2] Moreover, diagnosing isolated A. simus remains (such as femora, scapulae, certain vertebrae, ribs, podials) from brown bears can be challenging, as some large brown bears overlap in dimensions with small A. simus specimens.[7] Beyond standard differences between tremarctine and ursine bears, A. simus has a more anterior protocone and extended enamel ridge forming a shearing blade on the maxillary P4. The molars are also shorter and broader in Arctodus than brown bears.[21]
Evolution
| Tremarctinae within Ursidae | |||||||||||||||||||||||||||||||||||||||||||||
|
Arctodus belongs to the subfamily Tremarctinae, which appeared in North America during the late Miocene epoch in the form of Plionarctos. The medium-sized Arctodus pristinus, Tremarctos floridanus, and Arctotherium species evolved from Plionarctos in the Blancan age of North America.[2][22][23] The genetic divergence date for Arctodus is between 5.5 million and 4.8 million years ago,[19][20][24] around the Miocene-Pliocene boundary, when tremarctine bears, along with other ursids, experienced an explosive radiation in diversity, as C4 vegetation (grasses) and open habitats dominated. The world experienced a major temperature drop and increased seasonality, and a faunal turnover, which extinguished 70–80% of North American genera.[25][26]
Arctodus first appears in the early Late Blancan (Early Pleistocene), with the earliest finds being A. pristinus from the Kissimmee River 6 and Santa Fe River 1 sites in Florida,[1][2][27] dated from 2.6-2.3 Mya,[28] and Arctodus sp. from 111 Ranch (~2.6 Mya) and San Simon (about 2.2 Mya) in Arizona,[29][30][31] and La Union in New Mexico (Mesilla Fauna B, 2.2-1.8 Mya).[32] This appearance coincides with the start of the Quaternary glaciation, and the second phase of the great American biotic interchange, with the first records of the main South American faunal wave into the United States.[30]
Arctodus pristinus was mostly restricted to the more densely forested thermal enclave in eastern North America.[33][34] with the greatest concentration of fossils being in Florida.[2] During the early Irvingtonian faunal stage, a western population of A. pristinus evolved into the enormous A. simus, with the earliest confirmed records being at least 780,000 years old from the Irvington type locality in California.[7][35] Correspondingly, A. simus is most plentiful from western North America,[36][37] albeit preferring mixed habitat such as temperate open woodlands.[38][39][40][41][42][20] Their ranges may have met in the Middle Pleistocene of Kansas,[7] with A. simus migrating east in the Late Pleistocene (around the extinction of A. pristinus).[23][43] Although both Arctodus species co-inhabited North America for at least half a million years during the Middle Pleistocene (A. pristinus went extinct about 300,000 BP), no direct evidence of overlap or competition has been found in the fossil record as of yet, as both species established largely separate ranges.[23]
Irvingtonian-age (1,900,000-250,000 BP) specimens of A. simus are particularly sparse. Finds are mostly from California, with additional remains from Texas, Kansas, Nebraska, and Montana.[44][45][46] However, A. simus became a pancontinental species in the Rancholabrean faunal stage (Late Pleistocene), sharing that distinction with the American black bear.[35][41] Despite A. simus' large temporal and geographic range, fossil remains are comparatively rare (109 finds as of 2010, in otherwise well-sampled localities).[23][19]
Description
Size

Arctodus pristinus
Around the size of grizzly bears, A. pristinus specimens closely overlap the size of Tremarctos floridanus, with some males of A. pristinus overlapping in size with the females of A. simus.[1] Floridan A. pristinus individuals were calculated to an average around 140 kg (310 lb).[47][48] The dimensions of some individuals from Port Kennedy Bone Cave and Aguascalientes, though, suggest that northern and western A. pristinus populations may have been larger than Floridan A. pristinus,[7] being up to 400 kg (880 lb).[49]
Arctodus simus
Some A. simus individuals might have been the largest land-dwelling specimens of Carnivora that ever lived in North America. Standing up on its hind legs, A. simus stood 2.4–3.4 m (8–11 ft),[50][51] with a maximum vertical arm reach of 4.3 m (14.1 ft).[52] When walking on all fours, A. simus stood 1–1.67 m (3.3–5.5 ft) high at the shoulder, with the largest males being tall enough to look an adult human in the eye.[53][51][54][55] The average weight of A. simus was about 625 kg (1,378 lb), with the maximum recorded at 957 kg (2,110 lb).[56][47]
Sexual dimorphism

Arctodus has been described as very sexually dimorphic; A. simus males were sometimes twice as large as females.[57] Akin to its relative, the spectacled bear (with male spectacled bears being 30-40% larger than females), the larger, massive Arctodus individuals are considered male, particularly older males, with the smaller, more lightly built individuals being females.[23][57][44] As with T. ornatus, specimens with a large sagittal crest were likely male, whereas females had a reduced or no sagittal crests.[2] A 2025 mitochondrial DNA study affirmed sexual dimorphism in A. simus, with sexually dimorphic size classes and a uniform population being described from at least 31 individuals recovered from 28 sites across the United States and Canada.[58]
Studies
In a 2010 study, the mass of six A. simus specimens was estimated; half of the specimens weighed between 740 and 957 kg (1,631 and 2,110 lb), with a mean weight around 850 kg, suggesting larger (male) specimens were probably more common than previously thought. The other (female) specimens were calculated to be less than 500 kg (1,100 lb). The weight range calculated from all examined specimens was between 957 kg and 317 kg (699 lb).[56] A 1999 study by Per Christiansen calculated a mean weight of 770 kilograms (1,700 lb) from seven male A. simus limb bones, suggesting large males weighed between 700 and 800 kg (1,500 and 1,800 lb).[59] Hypothetically, the largest A. simus males may have approached 1,000 kg (2,200 lb),[59][60] or even 1,200 kg (2,600 lb).[49] However, a 2006 study argued that the maximum size of Arctodus was roughly 555 kg (1,224 lb), based on the largest known skull.[61]
Anatomy
The two species of Arctodus are differentiated not only by size, but also by the shorter snout, greater prognathism, more robust teeth, and longer limbs of A. simus, and the relative proportions of each species' molars and premolars. A. pristinus is distinguished from A. simus by smaller, narrower, and less crowded teeth. The morphologies of both species are otherwise very similar. As a result, differentiating A. simus from A. pristinus can be difficult, as male individuals of A. pristinus can overlap in size with females of A. simus.[23][62][63] Arctodus simus superficially resembled living hyaenids in skull shape and relative lengths of the trunk, back and limbs.[61] The most nearly complete skeleton of A. simus found in the United States was unearthed in Fulton County, Indiana; the original bones are in the Field Museum of Natural History, Chicago.[53][64]
Skull

Members of the Tremarctinae subfamily of bears appear to have a disproportionately short snout compared with most modern bears, giving them the name "short-faced". Arctodus has also been argued to exhibit a wide and shortened rostrum, potentially giving Arctodus a more felid-like appearance.[46][65] Matheus suggested that a broad snout could have housed a highly developed olfactory apparatus, or accommodated a larger throat passage to bolt down large food items, akin to spotted hyenas.[66] This apparent shortness, though, is an illusion caused by the deep snouts and short nasal bones of tremarctine bears compared with ursine bears; Arctodus has a deeper but not a shorter face than most living bears. This characteristic is also shared by the only living tremarctine bear, the omniherbivorous spectacled bear.[36][56][67] Snout deepness could be variable, as specimens from Huntington Reservoir in Utah, and the Hill-Shuler locality, Texas, were noted as being distinctly "short-faced" in comparison with other A. simus individuals.[68][69]
The orbits of Arctodus are proportionally small compared to the size of the skull, and somewhat laterally orientated (a characteristic of tremarctine bears), more so than actively predatory carnivorans or even the brown bear, suggesting that stereoscopic vision was not a priority.[61][67][70] The optic canal and other sphenoidal openings crowd together more in A. simus than in Ursus.[21] Although samples are limited, the middle ear bones of A. simus are proportionally larger than modern ursine bears, suggesting the species was particularly attuned to low-frequency sounds.[71] The canalis semicircularis lateral suggests that A. pristinus had a head posture of 48°, which being more oblique than several Arctotherium and Tremarctos species, could also infer a greater capacity for long-distance vision.[72]
Morphologically, Arctodus simus exhibits masticular characteristics common to herbivorous bears. This includes cheek teeth with large, blunt surface areas, a deep mandible, and large mandibular muscle attachments (which are rare in carnivorous mammals). As herbivorous carnivorans such as Arctodus lack the gut microbiota to efficiently break down plant matter, these features created a high mechanical advantage of the jaw to break down plant matter via extensive chewing or grinding.[36][26][73] Although the low mandibular condyle relative to the tooth row (and therefore potential wide gape) of Arctodus simus has been inferred as an adaptation for carnivory,[46][60][21] it is also present in the omniherbivorous spectacled bear.[56] Both A. pristinus and T. floridanus have condyles raised well above the plane of the teeth, although some A. pristinus specimens from Florida seem to also possess this feature.[8] The purpose of the highly vaulted calvarium and straight cheek bones of A. simus have been similarly disputed.[56] Michael Voorhies and Richard Corner argue that the jaws of A. simus are suited to deliver a strong bite at the canines, and calculated the force of the temporalis muscle of A. simus as being stronger than modern Ursus and canids, but equivalent to modern lions and Panthera atrox.[60]
A 2009 analysis of the mandibular morphology of tremarctine bears found notable differences between A. pristinus and A. simus, with A. simus specimens possessing a concave jaw, large masseter and temporalis muscles, deeper horizontal ramus, and a reduced slicing dentition length when compared to A. pristinus. Instead, A. simus was most similar to Arctotherium angustidens, but both species of Arctodus and Arctotherium angustidens were still comfortably in the "omnivorous" bear craniomorphotype.[67]
Dentition

The premolars and first molars of A. pristinus are relatively smaller and more widely spaced than those of A. simus.[1][23] As with other tremarctines, the features of the dentition can be quite variable, particularly the M2 molar.[2] An analysis of the Hunter-Schreger bands from the teeth of A. pristinus and A. simus demonstrated an evolutionary trend towards partially reinforced tooth enamel. This has been convergently evolved with giant pandas, agriotheriin bears, and Hemicyon.[74] The dentition of A. simus has been used as evidence of a predatory lifestyle, in particular the large canines, the high-crowned lower first molar, and the possible carnassial shear with the upper fourth premolar. However, the wearing of the molars to a relatively flat and blunt loph (suitable as a crushing platform as per modern omnivorous bears), small shear facet, and the flattened cusps across age ranges (unlike carnivores, which instead have carnassial shears) disagree with this hypothesis.[36][75][21]
Dentition can be a poor indicator of size in A. simus, as some medium-sized individuals have teeth that surpass the size of those with the largest skeletons. Additionally, while A. simus evolved from the smaller A. pristinus, their teeth remained generally the same size.[7] A specimen of A. simus from the Seale Pit of the Hill-Shuler locality, Texas, with only two premolars, crowding of the anterior premolar out of line, and a wider and shorter muzzle, was suggested to be an undescribed form of Arctodus.[69]
Postcranial
Limbs

Researchers have differing interpretations on the limb morphology of Arctodus. A comprehensive 2010 study concluded that the legs of Arctodus were not proportionally longer than modern bears would be expected to have, and that bears in general are long-limbed animals, obscured in life by their girth and fur. The study concluded the supposed "long-legged" appearance of the bear is largely an illusion created by the animal's relatively shorter back and torso. In fact, Arctodus probably had an even shorter back than other bears, due to the necessary ratio between body length and body mass of the huge bear.[56][76] Uther researchers, though, argue that the limb bones of A. simus are proportionally longer than those of other bears, leading to a "gracile" appearance. Although longer, the proportions still overlap with Ursus, and the limb bones are stouter than in the large-bodied felids (Panthera). Rather than for running, these elongated limb bones may have evolved for increased locomotor efficiency during prolonged travel.[77][55] This stiff-legged, swinging gait could have been similar to a polar bear's.[78] Some researchers suggest that proportionally longer limbs may be an adaptation for increased vision over tall ground cover in an open habitat, or were used in tearing and pulling down vegetation.[36][54]
Researchers also disagree when interpreting the humerus of A. simus.[77] Sorkin argued that the pronation of the forearm and the flexion of the wrist and digits, and more lightly muscled forelimbs, all of which are crucial to grasping a large prey animal with the forepaws, were probably less powerful in Arctodus than in either the brown bear or in Panthera. This is due to a weak medial epicondyle and reduced development of the pronator teres muscle.[61] The forelimb of Arctodus could have been in the early stages of cursorial evolution, being capable of more efficient and high-speed, straight-line locomotion (relative to extant bears), and was possibly more adept at pursuing large prey than polar and brown bears.[79] Other researchers argue that the epicondyles were still well developed, with this wide range of ulna rotation suggesting that forearms of Arctodus were powerful and could subdue large prey.[77] A 2013 examination of Rancho La Brean specimens found that they did not possess distally elongated limbs, which discredited cursoriality. Furthermore, the relatively broad humeral and femoral epicondyles were characteristic of diggers and polar bears, and suggested A. simus could have foraged for roots, tubers, and ground squirrels, and/or had developed forelimb muscles to immobilize moving prey.[80] The shape of the elbow joint, along with an well-developed medial epicondyle, which forms an angle with the condyle, and shallower olecranon fossa, would have given Arctodus a higher degree of forelimb dexterity. Originally evolved to facilitate arboreality, other researchers believe that the terrestrial Arctodus (along with Arctotherium and the giant panda) retained this characteristic to assist in foraging for vegetation.[24][36][81]
Paws
The paws (metapodials and phalanges) of Arctodus were characteristically long, slender, and more elongated along the third and fourth digits compared to ursine bears. Arctodus' paws were therefore more symmetrical than ursine bears, whose feet have axes aligned with the most lateral (fifth) digit. Also, the first digit of Arctodus was positioned more closely and parallel to the other four digits (i.e. with straight toes, Arctodus had less lateral splaying).[76][54]
This is potentially contradicted, though, by possible A. simus trackways from near Lakeview, Oregon, with strong toe splaying, three centrally aligned and evenly spaced toes at the front, and two almost perpendicular lateral toes (80° from the axis of the foot on either side). The trackways suggest that Arctodus had an oval-shaped, undivided pad on its sole, front paws that were slightly larger than its back paws, possessed long claws, and had its hindfoot overstep the forefoot when walking, like modern bears.[82] An additional A. simus paw print measuring 15 cm (5.9 in) long and 19 cm (7.5 in) wide has been recovered from White Sands National Park, New Mexico.[83][84] Some claw marks attributed to A. simus at Riverbluff Cave (as they were 4 m above the floor of the cave) were nearly 20 cm in width.[85][86]
The presence of a partial false thumb in A. simus is a characteristic shared with T. floridanus and the spectacled bear, and is possibly an ancestral trait. Absent in the Ursinae, the false thumb of the spectacled bear has been suggested to assist in herbivorous food manipulation (such as bromeliads, leaves, berries, tree bark and fruits, cactus fruits and pulp, palm hearts and fronds) or arboreality.[87][26][36]
Paleopathology
Beyond carbohydrate-associated dental pathologies present in the genus,[62][88][21] extensive pathologies have been preserved on the most nearly complete skeleton of Arctodus. The leading hypothesis suggests the Fulton County Arctodus specimen suffered from a syphilis-like (treponemal) disease, or yaws, based on the various lesions present.[53][89][90] The same individual records a pathological growth distorting the right humerus,[61] with abscesses noted between the molars and on both ulnae. Hypotheses include syphilis, osteoarthritis, a fungal infection in addition to long-term syphilis, or an infected wound.[53][91] Several specimens from Fairbanks, Alaska, also exhibit either pathological growths or periodontal disease,[7] along with a healed toe bone from Big Bear Cave, Missouri.[64]
Paleobiology
Locomotion
Paul Matheus proposed that A. simus may have moved in a highly efficient, moderate-speed pacing gait, more specialized than modern bears. His research concluded that the large body size, taller front legs, high shoulders, short and sloping back, and long legs of Arctodus also compounded locomotive efficiency, as these traits swelled the amount of usable elastic strain energy in the tendons, and increased stride length, making Arctodus built more for endurance than for great speed.[50][55] His calculations suggested that Arctodus likely had a top speed of 40–45 km/h (25–28 mph), and based on hyaenid proportions, would shift from single-foot locomotion to a pace at 8.5 km/h (5.3 mph), and would begin to gallop at 18.5 km/h (11.5 mph), a fairly high speed. Based on other mammals, the optimal pace speed of Arctodus would have been 13.7 km/h (8.5 mph). For comparison, hyenas cross country at 10 km/h (6.2 mph).[55] This mobility would have facilitated travelling across a large home range, which may have topped 1,000 sq mi (2,600 km2).[64] Swimming has also been presented as a hypothesis for the colonization of Vancouver Island by Arctodus simus.[92][64]
Maturity
Examinations on a mostly full-sized (likely 4- to 6-year-old female) individual of A. simus from an Ozark cave suggest that Arctodus, like other ursids, reached sexual maturity well before full maturity. Fused sutures, epiphyses, and epiphyseal plates, well developed premassateric fossa, along with tooth eruption and tooth wear, have been used to determine adulthood in Arctodus.[2][62][19]
Genetic diversity
Legend: 8px A. simus described by Salis et al. (2025)
8px Chiquihuite Cave A. simus
8px Unassigned A. simusSeveral mitochondrial DNA studies suggest Arctodus simus had a notably low level of genetic diversity, comparable with solitary & wide-ranging carnivorans such lynx and puma, or species with recent bottlenecks.[58][93] The lack of mitochondrial endemism demonstrated in a landmark 2025 study demonstrated that A. simus lived in a single interconnected population and was wide-ranging, which may be linked with its morphological adaptations towards long-distance travel. Like other fauna endemic to the pre-Pleistocene Americas, A. simus maintained genetic connectivity between its eastern Beringian population and populations south of the ice sheets until the isolation of Beringia during the Last Glacial Maximum, with four distinct lineages across five haplotypes intermingled with eastern Beringian specimens. The last common ancestor between Beringian and southern populations was 31,500 BP.[58]
While suggested by Kurtén in the past, there is no evidence for subspecies in A. simus, such as distinctive genetic diversity or phylogeographic structure. The last common ancestor between all specimens from the 2025 study was the Middle Pleistocene (209,100 BP); however, all specimens which postdate 100,000 BP have a last common ancestor was in the Late Pleistocene (73,600 BP).[58] However, this does not entirely preclude genetic diversity in Arctodus simus, with genetic samples from Chiquihuite Cave, Zacatecas possibly indicating a deep divergence with other specimens of A. simus.[19] A sample from the Channel islands have been studied but its relatedness is unknown.[94]
A 2020 analysis of the genetic history of three A. simus individuals from the Yukon suggests an extended history of small effective population size. A steady decline of the breeding population around 1 Mya (from about 16,500 individuals to 4,000 individuals) was halted by a slight increase in numbers 60,000 BP (7,500 individuals). This was followed by a decrease around 48,000 BP (correlated with expanding Yukon forests circa the MIS 3 interstadial), which continued until the local extinction of Beringian A. simus by near 23,000 BP during the last glacial maximum.[20]
Hibernation

Arctodus pristinus specimens have been found in caves such as Port Kennedy (Pennsylvania, where fossils from as many as 36 individuals have been found), Cumberland Cave (Maryland) and Hamilton Cave (West Virginia), often in association with the black bear. This suggests a close association with the biome.[2][95][96]
According to a 2003 study, in karst regions, fossils of A. simus have been recovered almost exclusively from cave sites.[62] In the contiguous United States, that about 38% of all sites are from caves (possibly 50% in western USA)[97] suggests a close association between this species and cave environments. Metabolic denning (hibernation/torpor) is unclear in Arctodus. Like polar bears, males and unmated females of A. simus may have forgone denning, leaving maternal denning by females as the preferred explanation behind the recovery of the small, yet relatively complete individuals recovered from caves.[62][48][58] However, to date, no records of adults with associated offspring are reported from caves.[57] Regardless, Arctotherium angustidens, a fellow giant short-faced bear, has been recovered with offspring from a cave in Argentina.[98]
At Riverbluff Cave, the most abundant claw marks are from A. simus. They are most abundant at the bear beds and their associated passageways, indicating a close relationship with denning.[85] Numerous "bear" beds often preserve A. simus and both Pleistocene and modern American black bears in association (U.a. amplidens and U. a. americanus)- such deposits have been found in Missouri, Oklahoma, and Potter Creek Cave, California. These mixed deposits are assumed to have accumulated over time, as individual bears (including Arctodus) died during winter sleep.[5][99][100] Furthermore, environmental DNA suggests that Arctodus and black bears shared a cave in Chiquihuite Cave, Zacatecas.[19] At Labor-of-Love Cave, Nevada, both American black bears and brown bears have been found in association with A. simus. A study in 1985 noted that sympatry between Arctodus and brown bears preserved in caves is rare, with only Little Box Elder Cave, Wyoming, and Fairbanks II, Alaska, hosting similar remains.[36][101]
Diet
Herbivory

The fact that Arctodus did not significantly differ in dentition or build from modern bears has led most authors to support the hypothesis that the A. simus was omnivorous, like most modern bears, and would have eaten significant amounts of plant matter.[75][102] Morphologically, A. simus exhibits masticular and dental characteristics that confirm that short-faced bears such as the spectacled bear and Arctodus were adapted to and actively consumed vegetation.[36][67][26][75][73] This is affirmed by a lack of dental damage associated with carnivory amongst specimens of Arctodus.[103] Dental pathologies that have been found, such as incisor wear and supragingival dental calculi in a young individual from Missouri,[62] and cavities associated with carbohydrate consumption in individuals from the La Brea Tar Pits and Pellucidar Cave (Vancouver Island), further suggest an omnivorous diet for A. simus.[88][103][21] Additional morphological adaptations include dexterous forelimbs and a partial false thumb, which would have assisted in foraging for vegetation,[81][87] along with the body size of large A. simus (about 1000 kg) matching or exceeding the expected upper limitations for a terrestrial carnivore (based on the more restrictive energy base for a carnivorous diet).[36][104][105]
While features of A. simus' morphology suggest herbivory, their close phylogenetic relationship to the omniherbivorous spectacled bear presents the possibility that these traits may be an ancestral condition of the group. A browsing diet foraged from the canopies of trees and shrubs could have been difficult with the large and flattened rostrum and incisor build of Arctodus, while evidence of digging adaptations in Arctodus' forelimbs and claws (e.g. for rooting) is mixed.[54][61][80] Regardless, gross tooth wear suggests consumption of plant matter in the diet of A. simus.[26][36] The diet of individuals from La Brea was most similar to the spectacled bear, which consumes tough leafy matter, seeded and pitted fruits, and occasional animal flesh. Arctodus' tooth wear remained consistent throughout the Pleistocene in La Brea. This indicated a less generalized diet than modern omniherbivorous black bears, with none of the dental evidence of hard food consumption (such as carcasses or nuts) found in polar bears, black bears, and hyenas.[26] Comparisons with the dental microwear of Ursus speleaus suggest dietary differences between the species, with cave bears consuming tougher vegetation than A. simus.[102] Although some researchers argue that herbivory should be more obvious from the isotope data gathered from northern Arctodus,[63] several Arctodus coprolites from The Mammoth Site in South Dakota and Meander Cave at Ni'iinlii'njik Territorial Park, Yukon contain Juniperus seeds (toxic to black and brown bears).[70][106]
Opportunistic carnivory

Evidence suggests that Arctodus also consumed meat, as evidenced by elevated nitrogen-15 isotope levels (corresponding to protein consumption) and bone damage on contemporary fauna. Additionally, elevated carbon-13 levels (corresponding to C3 resources) from many localities (Alaska,[108] California,[38][109] San Luis Potosí,[39] Texas,[110] Vancouver Island,[92][111] and the Yukon)[112] largely suggest browsers (and browsed vegetation) were the core of A. simus' diet.[56]
Arctodus simus' status as a predator is questioned by its gracility and lack of agility, which could have complicated predation upon adult megaherbivores, and hindered the chasing down of nimbler prey.[55][79] Nevertheless, larger A. simus males are suggested to have been more carnivorous than females, as very large brown bears may not be able to sustain themselves on a vegetarian diet.[111] Furthermore, the much larger frame of A. simus would have provided an advantage in disputes over carcasses.[23]
Studies establish that A. simus would have had a varied diet across its range,[26] and was outcompeted and/or more herbivorous with increased competition from other predators.[88][111][108][109] The extinction of cursorial, hypercarnivorous Borophagus and Huracan in the more open western North America left a vacant niche, possibly contributing to the evolution of A. simus (along with changes to the herbivore guild).[24][77]
Bone damage

The bite marks found on many bones of ground sloths (Northrotheriops texanus) and young proboscideans at Leisey Shell Pit in Florida matched the size of the canine teeth of A. pristinus. Whether these bite marks are the result of active predation or scavenging is unknown.[1] Additionally, A. pristinus was the most common large predator from Port Kennedy Cave, Pennsylvania, where the majority of mastodon remains were juveniles and likely represent accumulated prey.[113]
Arctodus simus has been found in association with proboscidean remains near Frankstown, Pennsylvania, (juvenile mastodon), and at the Mammoth Site, South Dakota (Columbian mammoths).[70][114] Importantly, the canines of Panthera atrox overlap in size with A. simus, complicating the identification of tooth marks.[63] However, a mammoth rib from the Mammoth Site has a probable canine puncture attributed to A. simus.[115] A woolly mammoth specimen from Saltville, Virginia was likely scavenged on by A. simus, as evidenced by a canine gouge through the calcaneus.[63] Several Columbian mammoth bones from a cave near Huntington Reservoir, Utah also record ursid gnaw marks attributed to Arctodus, with an Arctodus specimen preserved in association with the remains.[68] Damaged bones from near Tanana River, Alaska, suggest that Arctodus transported megafaunal long bones back to a cave-like den and chewed on them,[116][117] at a time when lions had a limited overlap with Arctodus in Beringia.[118][88] Spiral fractures on ungulate long bones from Red Willow, Nebraska may have also been caused by A. simus.[60] Furthermore, a perforated peccary ilium from Sheriden Cave has also been hypothesized as being scavenged by A. simus.[119] Bone damage on a cranial fragment (and possibly the humerus) of an Arctodus individual on Vancouver Island may have been due to cannibalism.[21][111]
Beringia
Analysis of bones from Alaska showed high concentrations of nitrogen-15, an isotope accumulated most strongly in carnivores. Although few specimens exist, no evidence of the same carbohydrate-related dental pathologies has been found in southern populations of A. simus.[88] Based on this evidence, A. simus was suggested to have been more carnivorous in Beringia than the rest of North America (with a preference for herbivores, which consumed C3 vegetation, particularly caribou).[108][112][120] Increased carnivory may be due to a lower proportion of competitors and probably a lower availability of carbohydrate-rich food supplies across the year in the far northern latitudes.[88] Survival during the cold season for some northern populations of A. simus could have depended on the regular scavenging of ungulate carcasses, as is the case with Alaskan brown bears.[56][109] Ultimately, an opportunistic foraging strategy including up to 50% vegetation, and the meat of reindeer, muskox, carrion, and possibly some predators, is consistent with the isotopic data and the conclusions of the ecomorphological studies.[108]
Carbon isotope studies
Although elevated nitrogen-15 levels have been argued to indicate carnivory, even the isotope data of the most carnivorous Beringian Arctodus overlapped with modern, typically omniherbivorous brown bears from Europe, eastern Wyoming, and central Montana, demonstrating that isotope data cannot distinguish between hypercarnivores and omnivores that eat a significant amount of animal matter.[61][108] Studies are also complicated by a lack of compound-specific data,[109] and isotope data being variable in carbon-13,[121][122] and nitrogen-15 (due to individual/evolving prey and plant choices, the isotopic composition of the local environment, and nutritional stress).[112][123] Carbon-13 levels in Arctodus simus (enriched by both plants and prey matter) consistently reflect a diet based on C3 resources, typically found in closed to mixed habitats with at least some tree cover (such as open woodlands).[38][39][92][108][109][110] This includes C3 vegetation (leaves, stems, fruits, bark, and flowers from trees, shrubs, and cool season grasses)[36] and the browsers that fed on them, such as deer, camelids, tapir, bison and ground sloths.[39][56]
| Location | Age | Carbon 13 (δ13C) | Nitrogen 15 (δ15N) | Results |
|---|---|---|---|---|
| Irvington, California | Early Pleistocene | −14.5 | N/A | Arctodus simus carbon isotope values from Irvington (along with Fairmead Landfill and McKittrick Tar Pits) are consistent with diet based on C3 resources.[38] |
| Fairmead Landfill, California | Middle Pleistocene | −11.9 | N/A | Initially proposed to consume Columbian mammoth, and large ungulates,[124] a 2015 study recalculated Arctodus' carbon isotope values to be closest to C3 vegetation consuming deer and mastodon.[38] |
| Cedral, San Luis Potosí | Late Pleistocene | −11.8 | N/A | This Arctodus individual had the strongest δ13C value of its local fauna. Arctodus' carbon isotope value was closest to values from the tapir and Hemiauchenia.[39] |
| Natural Trap Cave, Wyoming | -19 | 8.1 | A 2012 study found that the Natural Trap specimen had the lowest δ13C of the Pleistocene fauna (-13.1), leading to suggestions that seasonality & individual choices within omnivorous diets could result in extreme isotope data in certain teeth.[122] A 2024 analysis of the Natural Trap Cave retrieved the highest nitrogen-15 result of its locality; while mammoth was suggested to be dominant in its diet, the study suggests that Arctodus isotope data was noticeably regional, and may have had flexible diets.[125] | |
| Channel Islands, California | -17.9 | 13.2 | Nitrogen isotope signatures suggested a ~19% consumption rate of seals (along with bison and camels). Fossil was likely transported post-mortem from the mainland; a partial reliance on marine resources has been suggested to be as a result of a competitive carnivore guild on mainland California. The marine signal was in between island foxes and bald eagles, most closely resembling Late Pleistocene California condors.[109] | |
| McKittrick Tar Pits, California | -10.9 | N/A | This carbon isotope value was closest to deer, similar to the one inferred for the Cedral individual.[39][124] | |
| Little Box Elder Cave, Wyoming | -14.9 | N/A | Like the 2012 Natural Trap Cave analysis, the Little Box Elder Cave specimen had distinctly lower δ13C levels, being only higher than Ursus.[122] | |
| Friesenhahn Cave, Texas | -16.5 | 9.7 | The Friesenhahn Cave specimen had a nitrogen-15 sample closest to the omnivorous striped skunk.[110] | |
| Vancouver Island, British Columbia | -18.9 | 10.6 | A specimen from Cowichan Head, Vancouver Island, had isotopes suggesting a terrestrial diet at a relatively high trophic level.[92] | |
| Fairbanks, Alaska | -18.0 | 8.4 | Nitrogen & carbon isotope data from several specimens suggests that Arctodus specialized on reindeer in central Alaska, both before and during the Last Glacial Maximum.[112] | |
| Dawson, Yukon | -18.5 | 9.9 | Arctodus' nitrogen-15 levels are higher in the Yukon, suggesting a possibly even higher trophic level than other Arctodus in eastern Beringia. However, this contrast likely reflects subtle differences in the isotopic composition of local plants,[66][126] & muskox in the region, and possibly fellow predators and their kills, complimenting the consumption of reindeer.[108] |
Paleoecology
Arctodus pristinus

Endemic to the late Blancan faunal stage and Irvingtonian faunal stage, Arctodus pristinus was a relatively large tremarctine bear.[1] Sometimes referred to as the eastern short-faced bear,[127] A. pristinus has been found in Florida,[2] Kansas, Maryland,[7] New Mexico,[128] Pennsylvania,[97][95] South Carolina,[129] and West Virginia in the US,[7][96] and Aguascalientes in Mexico.[130] Possible remains have also been recovered from Arizona.[7][29] A. pristinus is particularly well known from Florida, especially from the Leisey Shell Pit.[131] Like A. simus and other tremarctine bears, A. pristinus had adaptations for herbivory, and was likely largely herbivorous itself,[2] although Arctodus has been suggested to be generally more carnivorous than contemporary bears.[1][46]
Eastern North America
Arctodus pristinus is considered a biochronological indicator for the period between the Late Blancan and late Irvingtonian periods of Pleistocene Florida- more fossils of A. pristinus are known from Florida (about 150) than anywhere else. A. pristinus recovered from Florida have intraspecific variation that has probable temporal and geographic origins.[2] In the Early Pleistocene of Blancan Florida, the Santa Fe River 1 site (~2.2 Ma), which Arctodus pristinus inhabited,[1][2] was a fairly open grassland environment dotted with karst sinks and springs and dominated by longleaf pine flatwoods. Arctodus pristinus co-existed with terror birds, sabertooth cats, giant sloths (Eremotherium, Megalonyx, Paramylodon), giant armadillos (Glyptotherium, Holmesina, Pachyarmatherium), gomphotheres, hyenas, canids (Borophagus, Canis lepophagus), peccaries, llamas, dwarf pronghorns, and three-toed horses. Smaller fauna included condors, rails, ducks, porcupines, and alligators.[132][133]
Arctodus simus
Evolving from the smaller A. pristinus in the early Irvingtonian faunal stage,[7][35] scholars today mostly conclude that A. simus was a colossal, opportunistic omnivore, with a flexible, locally adapted diet akin to the brown bear.[56][67][21][109][47] If A. simus wasn't largely herbivorous,[2][36] the scavenging of megaherbivore carcasses, and the occasional predatory kill would have complimented the large amounts of vegetation consumed when available.[56][61][21][134]
Sometimes referred to as the bulldog bear,[135][136] or great short-faced bear,[17][137] Arctodus simus has been recovered from a comparatively small number of finds in relation to other large carnivorans, with the species suggested to have lived in low population densities.[19] Matheus argues that unlike other Nearctic carnivorans, A. simus did not appear to have an ecological equivalent ("super-huge bear") in the Palearctic realm.[138]
Arctodus simus was initially restricted to the western United States during the Irvingtonian.[44][45][46] However, in the Rancholabrean faunal stage, A. simus expanded its range from southern Canada to central Mexico in the west, and to Pennsylvania and Florida in the east.[7][14][23][139] A. simus also inhabited eastern Beringia at times, with finds today spanning from northern Alaska to the Yukon.[19][23][139] Based on the wide distribution of the species, A. simus inhabited a variety of climatic conditions and environments.[23][36][88] A 2009 study examining megafaunal extinctions in Northern America noted 12 records (<40,000 BP) of Arctodus simus from the Intermontane Plateaus, 7 from the Pacific Mountain System, 6 each from the Interior Plains and Interior Highlands, 3 each from the Atlantic Plains and Rocky Mountain System, and 1 from the Appalachian Highlands.[140]
A. simus was relatively plentiful in western North America, with over 50% of specimens from the western contiguous United States (<40,000 BP).[41][88] Arctodus simus was integral to what has been referred to as the Camelops fauna, or alternatively Camelops/"Navahoceros" fauna, a faunal province centered in western North America. The Camelops fauna was also characterized by shrub-ox, prairie dogs, dwarf pronghorns, Shasta ground sloths, and American lions. The diverse flora of the Camelops faunal province included montane conifers and oak parklands, shrub and grassland that stretched across the North American Cordillera south of Canada, to the Valley of Mexico. This faunal province supported a variety of large grazing and browsing mammals.[37][141][142]
Western Mountains

The Pacific Mountain System seems to represent a cradle of evolution for Arctodus simus. The earliest confirmed finds of A. simus are from Irvington, California,[143] which are at least 780,000 years old, but may be older than 1.2Mya.[35] Other Irvingtonian age sites come from California, such as Elsinore,[144][145] Fairmead,[146] and Murrieta.[44] Older yet disputed remains come from El Casco (1.4Mya).[44][147]
Despite the shift to aridified, mixed C3-C4 habitats between the Early and Late Pleistocene of the Central Valley (~1Mya to ~15,000 BP), Arctodus simus remained consistent with the consumption of C3 resources. Dire wolves and Arctodus simus were ever present members of the local predator guild throughout the Pleistocene, whereas jaguars, Homotherium, Miracinonyx and Smilodon (Fairmead & Irvington) transitioned to Panthera atrox and coyotes (McKittrick Tar Pits).[38] Although Arctodus could have hunted other closed habitat browsers such as deer (Cervus & Odocoileus), camelids (Hemiauchenia & Camelops), Paramylodon, and peccaries,[38] specimens collected from the La Brea Tar Pits suggest A. simus preferred a herbivorous diet.[26] However, nitrogen-15 samples from Rancho La Brea varied across A. simus individuals, with some specimens being in the same trophic level as Smilodon fatalis.[148] A. simus is particularly famous from fossils found in the La Brea Tar Pits, with 33 individuals recovered (the most of any locality).[148][149][150] As only one juvenile has been found from La Brea, A. simus is suggested to have been solitary.[134] Many more finds come from across California,[7][5][151] Vancouver Island,[21][92] and Washington,[92] where the semi-arid woodland/scrub transitioned to forest-steppe,[152] and open grasslands/heath.[92]
Comparatively, the Rocky Mountain System had the fewest number of specimens of Arctodus simus in western North America.[41] However, one of the youngest dated A. simus is from a cave near Huntington Reservoir, Utah, which sits at an elevation of 2,740m (~9,000 ft). The central and southern Rocky Mountains may have acted as refugia for boreal parkland megafauna from the plateau such as A. simus,[68][37] with the Huntington specimen being the only confirmed extinct megafauna dated to the Younger Dryas of the Great Basin.[153] Other remains have been found from Wyoming (such as Natural Trap Cave),[154][155][122][125] and Montana.[156][157]
Intermontane Plateaus

The Intermontane Plateaus had the highest number of Arctodus simus specimens south of the ice sheets.[41][140] The region has yielded some of the largest specimens of A. simus, including what was once the largest specimen on record, from Salt Lake Valley, Utah.[158] Disputed Irvingtonian remains from eastern California (Victorville and Vallecito Creek) may be as old as 2Mya.[7][159][160][161]
In contrast with other parts of North America, the plateaus received more rainfall during the Late Pleistocene, as glacially cooled air collided with hot desert air. As a result, this greatly expanded the range of subalpine parkland, piñon-juniper & ponderosa woodlands, sagebrush grasslands and pluvial lakes where desert exists today.[153][152][97][162] The mid-Wisconsian U-Bar Cave, New Mexico, was vegetated by sagebrush, grasses, and woodlands. Notable fauna which lived alongside Arctodus simus included Shasta ground sloth, shrub-ox, pronghorns (Stockoceros, Capromeryx), Camelops, Odocoileus, horses, Lynx, puma, black bear, mountain goats, prairie dogs, and Stock's vampire bat.[40][163] Dire wolves were also found in association with Arctodus simus, and both species are the most common large carnivorans of Rancholabrean New Mexico.[97] Beyond Utah and New Mexico,[97][164][165][166][167][168] other important US specimens have also been found in Arizona, eastern California,[7][169][170] Idaho,[7] Nevada,[171] and eastern Oregon.[172][173][174]
The Intermontane Plateaus extended into central Mexico, with the Mexican Plateau sharing the Late Pleistocene mesic savanna and piñon–juniper woodland ecoregion with the southwestern USA.[175][176] While Arctodus was limited to the Mexican plateau, the typical tropical thorn scrub and scrub woodland of the plateau was seemingly prime habitat for tremarctine bears.[41][152][177] An Arctodus simus individual from Cedral, San Luis Potosí, inhabited closed vegetation, based on the individual's δ13C signature. Consuming C3 resources, its diet may have incorporated local C3 specialists such as tapir, llamas, camels, and Shasta ground sloth along with browsed vegetation. The site, incorporating trees, herbs and cacti, hosted an open gallery forest near grassland or scrub with a humid climate.[39] Similar highland remains have been recovered from Jalisco,[178] Michoacán,[177] Puebla,[7] State of Mexico,[179][180] and Zacatecas.[19]
Interior Plains
The Interior Plains were composed of temperate steppe grassland,[152] and among the specimens yielded from this region is one of the largest Arctodus simus currently on record, from the banks of the Kansas river.[181] The late Irvingtonian Doeden gravel pits in Montana preserves an open grassland habitat, with riparian woodlands, and likely some shrublands.[182] A. simus co-existed with ground sloths (Megalonyx, Paramylodon), Pacific mastodon, camels, and Bootherium.[183][184][45] As bison were yet to migrate into North America, Columbian mammoths and horses dominated these early Illinoian grasslands.[185] Additional Irvingtonian remains are from Kansas, Nebraska and Texas.[44][46][7]

In the Rancholabrean age, Arctodus simus, grey wolves and coyotes were part of a predator guild throughout the great plains, and were joined by Columbian mammoths, camels, Hemiauchenia, and American pronghorns. While the northern plains aridified into cold steppe (e.g. Mammoth site, South Dakota),[186] the southern plains were a parkland with riparian hackberry forests, and large expanses of mixed grass prairie grasslands grading into wet meadows, with limited seasonality. In the south (Lubbock Lake, Texas), this fauna was joined by Smilodon, dire wolves, grey fox and red fox, preying upon prairie dogs, horses (Equus & Haringtonhippus), peccaries, Odocoileus, Capromeryx, Bison antiquus and Holmesina.[186][187] Beyond Texas,[188] Arctodus has also been found in Iowa,[189] Kansas,[7][190] Nebraska,[7] and southern Canada (Alberta & Saskatchewan),[191][192][193] which when unglaciated, would have formed a tundra ecosystem with an ice-free corridor to Beringia.[194]
In the lowlands of the eastern Interior plains, the plains transitioned to closed habitat. At the terminal Pleistocene Sheriden Cave, Ohio, a mosaic habitat consisting of marsh, open woodland, and patchy grassland was home to Arctodus simus, Cervalces scotti, caribou, peccaries (Platygonus, Mylohyus), giant beaver, porcupine, and American pine marten.[195][119] Similar remains have been found in Indiana,[53] and Kentucky.[196][197]
Interior Highlands
To the south, the Interior Highlands had a very high density of Arctodus simus specimens (second only to the black bear),[41][88] due to the high rate of preservation in the cave-rich region. Sympatry between the two species is most apparent in Missouri; A. simus has been found in association with black bears at Riverbluff, Bat and Big Bear caves.[198] Big Bear Cave preserves fossilized hair associated with Arctodus.[62] During the Last Glacial Maximum, both bears were joined by dire wolves, coyotes, jaguars, snowshoe hare, groundhogs and beavers at Bat Cave, which also records thousands of Platygonus remains. These fauna inhabited well-watered forest-grassland ecotone with a strong taiga influence, although the region did occasionally cycle through drier, grassier periods. These open woodlands were dominated by pines and spruce, and to a lesser extent by oaks.[199][200][201][202][203] Additional finds have been recovered from Oklahoma.[99][100]
Eastern USA

Compared to other regions, Arctodus simus was relatively rare in eastern North America.[23][41][88] To the north, the Appalachian Highlands were dominated by taiga.[152] Post-LGM Saltville, Virginia, was a mosaic of grassy/herb laden open areas intermixed with open canopy boreal woodlands (oaks, pines, spruce, birch, firs) and marshes. Inhabiting in this C3 resource dominated environment were Arctodus simus, mastodon, (southernmost) woolly mammoths, Bootherium, horses, caribou, Megalonyx, dire wolves, beavers, Cervalces, and a variety of warm-adapted reptiles, suggesting a more mesic and less seasonal climate than today. Heavy bone damage on a mammoth carcass by both dire wolves and Arctodus suggests a potentially competitive scavenging relationship [204][63] Beyond Virginia,[57] additional remains have been found in Pennsylvania.[7][114][205]
To the south, the subtropical Atlantic Plains covered a great expanse of lowland, from the open deciduous woodlands of the Atlantic coast, to the semi-arid woodland/scrub of Florida, to the spruce-fir conifer forests and open habitat of the Gulf Coastal Plain. Although scarce, this contrast of habitats highlights the adaptability of Arctodus simus. At the Rainbow River and Lake Rousseau localities in Rancholabrean Florida, three Arctodus simus specimens have been recovered, alongside Smilodon, dire wolves, jaguars, ground sloths (Megalonyx, Paramylodon), llamas (Hemiauchenia, Palaeolama), Vero's tapir, giant beaver, capybara, Holmesina, horses, Bison antiquus, mastodon, Columbian mammoths and T. floridanus, in a climate similar to today's. Furthermore, the abundance of black bears, and particularly T. floridanus in Florida, has led to a theorized niche partitioning of ursids in Florida, with T. floridanus being herbivorous, and black bears and A. simus being omnivorous, with Arctodus being possibly more inclined towards carnivory.[23] Additional finds of south-eastern A. simus are from Alabama,[206] Arkansas,[207] Mississippi,[208][209][210] South Carolina,[211] and Texas.[69][212]
Beringia

Largely isolated by the Cordilleran and Laurentide ice sheets, Beringia is considered ecologically separate to the rest of North America, being largely an extension of the mostly open and treeless Eurasian mammoth steppe.[213] However, the occasional opening of an ice-free corridor, and the migration barrier of the Beringian gap, meant that eastern Beringia (Alaska and the Yukon) supported a unique assemblage of fauna, with many endemic North American fauna flourishing.[78] Currently, all specimens of A. simus in Beringia have been dated to a 27,000 year window (50,000 BP - 23,000 BP) from eastern Beringia,[118][21][7][64] while additional undated remains may be of Sangamonian age.[214][138] Unlike contemporary Beringian carnivorans, A. simus apparently never inhabited western Beringia (and therefore Asia).[138] The largest known skull of A. simus was recovered from the Yukon, and may represent the largest specimen known.[61][215]
The North Slope of Alaska <40,000 BP (Ikpikpuk and Titaluk rivers) preserves an upland and floodplain environment, with horses, bison then caribou being the most populous herbivores, and woolly mammoths, muskox, elk and saiga antelope more scarce. Cave lions, bears (Ursus arctos and Arctodus simus), and Beringian wolves made up the megafaunal predator guild.[216][217] Isotope data implies that caribou and muskox were principal components of the carnivorous portion of A. simus' Arctic diet, suggesting that the warmer, wetter vegetation on the margins of the dry mammoth steppe (similar to the moist acidic tundra vegetation which dominates today) was the preferred habitat of Arctodus in Beringia.[108][216]
Additionally, upon the flooding of the Bering Strait and expansion of moist tundra and peatlands in eastern Beringia during MIS-3, lions, brown bears and Homotherium went regionally extinct ~35,000 BP, whereas wolves and Arctodus persisted. Simultaneously, most megafaunal herbivores in Beringia experienced population bottlenecks, whilst mammoth populations steadily declined. This restriction of prey and habitat could explain the extinctions. However, genetically distinct cave lions and brown bears appear in MIS-2 circa the extinction of Arctodus in a re-emerged Beringia ~23,000 BP, opening up the possibility that some level of competition was at play.[118][108][218][219][220] The idea that Arctodus had a kleptoparasitic relationship with wolves and Homotherium in Beringia has been explored,[108] with the additional possibility that Arctodus successfully competed against brown bears and Homotherium for access to caribou pre-LGM.[112]
The local extinction of Arctodus in Beringia ~23,000 BP (possibly due to sharp climatic cooling associated with Heinrich Event-2),[118][21] was much earlier than in other parts of its range. While recolonized by cave lions and brown bears from Eurasia, Arctodus did not repopulate Beringia once the ice-free corridor to the south re-opened later in the Pleistocene.[118][221]
Map of fossil localities
Legend: 8px A. pristinus (Late Blancan / Irvingtonian)
8px Early/Middle Pleistocene (Irvingtonian) A.simus
8px Late Pleistocene (Rancholabrean) A. simus
8px Radiocarbon dated A.simus (14C Date (1σ), ≤50,000 BP)Relationships with other bears
Arctodus pristinus
In the Early Pleistocene, Arctodus pristinus was much more populous the south-east of North America, whereas the black bear was more common in the north-east.[222] The black bear has inhabited North America since at least the Middle Pleistocene,[111] while Tremarctos floridanus, a tremarctine bear inhabiting western North America at the time, is very similar to A. pristinus in terms of size, skeletal anatomy, and dietary preferences.[2]
Despite this, generally speaking large tremarctine fossils from the Early and Middle Pleistocene of Florida are considered to be A. pristinus, whereas those from the Late Pleistocene of Florida are considered to be T. floridanus. Indeed, black bears and T. floridanus are believed to have only colonized Florida with the extinction of A. pristinus (both of which only appear in Florida in the Late Pleistocene), however, T. floridanus could yet still be found from older sites in Florida.[2] T. floridanus was possibly an ecological replacement of A. pristinus, with T. floridanus finds being widespread in Rancholabrean Florida and the wider southeastern United States.[2][34][41]
Arctodus simus

The most commonly accepted ecological parallel of Arctodus simus in scientific literature is the brown bear.[56][67][21] Both brown bears and A. simus exhibit a high degree of dietary variability, and while largely herbivorous, meat can be an important dietary element to certain populations of both species.[108] Additionally, the potential of habitual kleptoparasitism is often noted in Arctodus, with brown bears being opportunistic, curious, and regularly stealing kills from smaller predators.[76][108] One past theory behind the extinction of A. simus is that A. simus may have been out-competed by brown bears as the latter expanded southwards from eastern Beringia ~13,000 BP, and gradually established itself in North America.[101]
However this has been refuted as new dates establish an extended coexistence, with some isolated A. simus remains being re-evaluated as brown bears.[118][21] Brown bears (along with lions, bison and red foxes) first emigrated to North America via Beringia during the Illinoian Glaciation, with brown bears first arriving between ~177,000 BP and ~111,000 BP in eastern Beringia. Genetic divergences suggest brown bears first migrated south during MIS-5 (~92,000 - 83,000 BP) upon the opening of the ice-free corridor,[118][111] with the first fossils being near Edmonton (26,000 BP). On a continent-wide scale, although the brown bear and A. simus were sympatric at times as brown bears spread into North America, A. simus may typically have dominated competitive interactions, and displaced brown bears from specific localities.[21] For example, brown bear at the La Brea Tar Pits only postdate Arctodus.[223][101][224] Additionally, Arctodus' prolonged co-existence with black bears may have put significant constraints on the black bear's evolution.[17]
At the end of the Pleistocene, one reason brown bears persisted where Arctodus simus went extinct was because Arctodus may have been less flexible in adapting to new and rapidly changing environments that impacted the availability or quality of food and habitat.[21] Brown bears and Arctodus have been discovered together in Alaska (then Beringia) between 50,000 BP and 34,000 BP,[118] and in later Pleistocene deposits in Vancouver Island, California, Wyoming and Nevada.[7][21][101]
Beringia
Isotope values (δ13C and δ15N) in numerous Beringian Arctodus simus specimens suggests A. simus usually occupied a higher trophic level compared with invading brown bears. While some Beringian brown bears consumed salmon, data from Beringian specimens of Arctodus clustered much more tightly, and suggested that only terrestrial sources of meat were important for Beringian Arctodus.[66] The forcing of a smaller bear into a more herbivorous diet has been compared to the modern relationship between brown bears and American black bears.[76][108] Where they overlap, black bears take the lower trophic niche, with lower population densities, much smaller territorial ranges, and seasonal migrations.[111] That A. simus (along with local climate change) may have excluded brown bears from eastern Beringia from ~34,000 to ~23,000 BP further suggests that Arctodus may typically have been dominant over brown bears.[218][219] When Arctodus went extinct in Beringia ~23,000 BP, brown bears recolonized Beringia, but had more carnivorous diets than their Beringian kin pre ~34,000 BP. This bolsters the idea that these bears competed for similar resources and niches.[118][21] Extinction and repopulation is further evidenced by the high genetic (mitochondrial) diversity of Beringian brown bears in contrast with Beringian A. simus. This contrast in genetic diversity has also been hypothesized to suggest that while female brown bears have a permanent home range, female A. simus may not have.[58][93][118] Despite their extended co-existence, genetic studies on Yukon A. simus suggest that no interbreeding occurred between A. simus and brown bears.[20]

Vancouver Island
Brown bears, black bears and Arctodus simus all co-existed on Vancouver Island once the island de-glaciated ~14,500 BP.[111][21] According to an isotope analysis, all three bears relied on terrestrial resources, Arctodus holding an intermediate trophic position between the brown and black bears. This may be an underestimate, as the Arctodus specimens from Vancouver Island are believed to be female; as per brown and black bears, female A. simus may have had a significant decrease in protein consumption compared with male A. simus when co-existing with brown bears. Additionally, an analysis of Arctodus' data suggested that when consuming protein, meat was preferred.[111] While niche-partitioning on Vancouver Island was possible, both A. simus and brown bears appeared to have preferred more open habitats.[111]
Convergent evolution
Both giant short-faced bears Arctodus simus and Arctotherium angustidens reached huge body sizes, in an example of convergent evolution.[24] However, beyond gigantism, there are notable differences between the species. Not only did Arctotherium angustidens reach a higher maximum weight (an exceptional specimen was calculated at ~1,670 kilograms (3,680 lb)), A. angustidens was a much more robust animal, in contrast with the gracile Arctodus simus.[49] Excluding the exceptional specimen, Arctotherium angustidens had been calculated to a weight range between 1,200 kilograms (2,600 lb) and 412 kilograms (908 lb),[225][56] with the largest specimens of either species being said to be comparable to one another.[225][67] The panda-relative Agriotherium africanum has also been suggested to share ecomorphological convergences with Arctodus simus.[61] Together with great size, the two species converged on several adaptations, including a skull with a short broad rostrums, premasseteric fossa on the mandible, possible carnassial shears (P4 and m1), and long limbs (relative to body length). These features were also shared by other extinct bears (Agriotherium, Huracan and Arctotherium bonariense).[61] However, while Agriotherium and Huracan have definitive adaptions for meat-heavy diets stemming from a running, predatory lifestyle, Arctodus simus lacks similar adaptations beyond proportionally longer limbs.[77]
Interactions with humans

One documented interaction with Clovis people is present at the Lubbock Lake Landmark, Texas. A likely already deceased Arctodus simus was processed for subsistence (butchery marks indicated skinning, de-fleshing and disarticulation) and tool production, much in the same way as a mammoth carcass (~13,000 BP / 11,100 14C BP ).[226][227] Additionally, other remains of the A. simus have been found in association with Paleo-Indian artifacts in Sheriden Cave, Ohio,[195][119][228] and Huntington Dam, Utah,[68] with an A. simus footbone fragment from Spalding, Idaho also being charred.[229][230] The direct relationship between humans and some associated Arctodus remains has been debated.[231][232][233] Human hunting and butchery of large megafauna, particularly mammoths and mastodon, would likely have put people in competition with A. simus. Defense against these large bears and the abandonment of carcasses are plausible outcomes,[21] along with the possible caching and disposal of carcass remains underwater to mask its odor from Arctodus.[234]
Migration barrier hypothesis
In the late 1980s, Val Geist hypothesized that "specialist, aggressive, competitive Rancholabrean fauna" such as Arctodus simus were a barrier for humans (along with other Siberian megafauna such as moose, grey wolves and brown bears) when migrating into North America (both Beringia and below the ice sheets).[235] Male A. simus were the largest and most powerful carnivorous land mammals in North America, with the potential specialization in obtaining and dominating distant and scarce resources. Humans in this hypothesis, though familiar with brown bears, would not have been able to avoid predation or effectively compete with A. simus and other large Pleistocene North American carnivores, making human expansion difficult in Beringia and impossible south of the ice sheets.[21][76][138] However, this theory has never been accepted by anthropologists. Paul Matheus argues that there were negligible ecological differences across the mammoth steppe, and that humans successfully competed against and even hunted territorial cave bears, cave hyenas, cave lions, leopards, tigers and wolves in Eurasia before reaching eastern Beringia, making the solitary Arctodus an unlikely impediment to expansion.[138] Indeed, new dates establish an extended co-existence of humans and megafauna such as Arctodus across North America.[236][237][238][239]
Beringia

Humans migrated to North America via the Siberian mammoth steppe, arriving at eastern Beringia (Alaska and the Yukon). However, the migration was halted at the North American Ice Sheet, which separated Beringia and southern North America for most of the Late Pleistocene.[240] Both humans and A. simus are first dated to ~50,000 BP in Beringia, both from sites in the Yukon, and co-existed until A. simus went extinct in Beringia ~23,000 BP during the Last Glacial Maximum. This co-existence continued through the regional extinction of other Beringian predators such as cave lions, brown bears and saber-tooth cats.[118] Important sites of pre-LGM human occupation in Beringia include Old Crow Flats and the Klondike,[241][242] Kuparuk River Valley,[243] and the Bluefish Caves.[244][245]
Contiguous North America
The human colonization of North America south of the ice sheets further disproves the idea that Arctodus was a migration barrier. The earliest universally accepted pre-Clovis site south of Beringia are the White Sands footprints in New Mexico, dated to ~22,000 cal. BP.[236] Other pre-LGM sites across the Americas, such as Chiquihuite Cave,[246][237] Valsequillo,[247] El Cedral,[248] Santa Elina,[238] Gault,[249] and Hartley Mammoth Site,[250] affirm that humans proliferated alongside megafauna (such as Arctodus) in southern North America for more than ten thousand years.[237][239][249][250][251] Humans were definitively widespread across the Americas by at least 15,000 BP.[21][239]
Extinction
Arctodus pristinus
Arctodus pristinus went extinct in the Middle Pleistocene (300,000 years ago),[23] being last recorded from the Coleman 2A site, Florida.[252] The evolution of Arctodus simus, competition with Tremarctos floridanus and black bears, and possibly the transitioning of Pleistocene Florida from a hot, wet, densely forested habitat to a still hot, but drier and much more open biome are thought to be factors behind the gradual disappearance of A. pristinus in the late Irvingtonian faunal stage.[23][41] There are dubious records of A. pristinus in South Carolina and California from the Late Pleistocene,[8][253] however these are heavily disputed.[129][109] Modern research establishes A. pristinus as existing between the Pliocene-Pleistocene boundary and the Middle Pleistocene.[2][23][129]
Arctodus simus
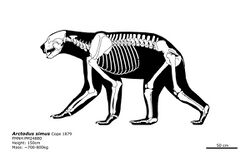
With the extinction of Arctodus pristinus, A. simus became the final representative of the genus. A simus went extinct around 12,800 years ago, and is one of the most recently dated megafauna to go extinct in North America, being reliably dated to within the Pleistocene-Holocene boundary (13,800 BP - 11,400 BP).[254][140][255] Both local and regionalized dietary flexibility has been a factor suggested for the species' longevity.[88]
Various factors, including the depletion in number of large herbivores,[223][54] the diminishing nutritional quality of plants during climate change, and competition with fellow omnivores (humans and brown bears) for food resources, have been suggested as the cause of Arctodus simus' extinction.[226] However, multiple studies put doubt on brown bears being culpable in A. simus' extinction, with the brown bear being more of an ecological replacement that was more adaptable to change.[41][21][54] Moreover, there is no systematic evidence that humans hunted large extinct Pleistocene carnivores in North America, and no clear indication of direct human involvement in the extinction of A. simus.[21] Additionally, dental wear evidence from Rancho La Brea does not suggest that food shortages were to blame for the demise large bodied carnivorans such as A. simus.[26]
Climate change
Of the factors discussed, vegetation shifts in the latest Pleistocene may have been particularly unfavorable for Arctodus simus, due to a reduction of quality foraging for subsistence. For example, on Vancouver Island (~13,500 BP), vegetation changed rapidly from open woodlands with abundant lodgepole pine to increasingly closed forests with shade-tolerant spruce, mountain hemlock, and red alder. These changes, effective by ~12,450 BP, point toward cool and moist conditions during the Younger Dryas stadial. Closed forests continued to expand in the early Holocene. Even though A. simus was not restricted to open areas and could inhabit in different environments, the timing of the regional shift from an open pine woodland habitat to a densely forested vegetation implies that these vegetation changes contributed to the local extinction of A. simus, along with many other megafauna.[21]
Low genetic diversity

A. simus had a low level of genetic diversity from most sampled specimens,[58][93] with a genetic study suggesting an extended history of low effective population size.[20] A loss and/or replacement of mitochondrial DNA lineages before the Last Glacial Maximum, and decrease in population size from a previously genetically diverse population, has been noted in a variety of Eurasian and American Late Pleistocene megafauna.[93][256] That the southern specimens were very closely related to Beringian specimens may further support this idea, as these populations had been isolated from before the Last Glacial Maximum (last common ancestor - 31,500 BP).[93][58]
A lack of genetic diversity has been attributed to a reduced ability to adapt to environmental conditions. Small population sizes may be characteristic of tremarctine bears- the spectacled bear, while having low levels of genetic diversity, has no signs of a recent genetic bottleneck. However, brown bears had diverse, sympatric source populations in Eurasia, allowing for repopulations/reinvasions into the Americas.[93] If Arctodus simus experienced genetic bottlenecks or local extinctions prior to the Last Glacial Maximum, A. simus would have been unable to supplement their reduced genetic diversity with new migrants like the brown bear could, making them vulnerable to extinction.[93]
Last dates
The youngest date for A. simus is circa 12,700 BP from Friesenhahn Cave, Texas, calibrated from 10,814 ± 55 radiocarbon years (14C BP). However, this date should be viewed with caution, as analyses suggest the collagen protein was degraded. A vertebra from Bonner Springs, Kansas, was dated to ca. 12,800 BP (based on 10,921 ± 50 radiocarbon years) from well preserved collagen. However, the same vertebra was previously assigned a younger date of ca. 10,980 BP (9,630 ± 60 radiocarbon years) from a different laboratory, which widens the possible age of this vertebra to between 9,510 and 11,021 14C BP (at 2σ).[57][257] Nevertheless, a specimen from Huntington Dam, Utah was also dated to ca. 12,800 BP from two radiocarbon dates (10,870 ± 75 & 10,976 ± 40 14C BP) and is therefore considered reliable.[57][254]
History of research
Subspecies hypothesis

Size differences between specimens of Arctodus simus (such as skull and long bone dimensions) led Kurtén to propose two forms; a larger subspecies emerged in the Irvingtonian (A. s. yukonensis), which was then displaced in the south by a smaller subspecies (A. s. simus) during the Rancholabrean epoch.[46][7][56] Other theories, such as the sexual dimorphism and individual variation known from Tremarctine bears, potential ecomorphs, and the overall lack of finds were additional factors used to explain the significant variation in Arctodus.[2][7][158]
However, sexual dimorphism has been described in A. pristinus.[2] Additionally, the presence of very large southern A. simus specimens (in California,[7][44][148] Florida,[23] and New Mexico)[97] and notably small northern specimens (Yukon and Vancouver Island)[19][21] already put doubt on this designation,[138] with the low number of specimens and sex-biased sampling potentially leading to perceived ecomorphologies.[23] For example, none of the specimens assigned to the larger morph (A. s. yukonensis) is from a cave passage, being usually isolated remains from open sites. Furthermore, over 70% of the smaller specimens (once assigned as the A. s. simus subspecies) are from cave deposits where bacula (penis bones) would likely be found if present, suggesting that mostly female individuals of A. simus were using caves.[62][48][7] The only baculum currently known from A. simus may belong to a black bear (Potter Cave),[64] while DNA evidence currently only affirms female specimens as having been recovered from caves.[58] Sexual dimorphism also explained why Arctodus teeth (from multiple individuals at the same site) generally clustered into two sizes.[2][7] While Rancho La Brea (the locality with the highest number of A. simus specimens) is the only site to preserve both size classes, radiocarbon dates confirm both sizes coexisted temporally, and were therefore sexes.[148] A 2025 mitochondrial DNA study found sexually dimorphic size classes and a uniform population from at least 31 individuals (derived from 28 deposits across the United States and Canada), further affirming sexual dimorphism in A. simus.[58]
"Super predator" hypothesis
One past proposal envisaged Arctodus simus as a brutish predator that overwhelmed very large but slow megafauna with its great physical strength.[55] However, despite being very large, its limbs were too gracile for such an attack strategy,[55][79][138] significantly more gracile so than Arctotherium angustidens at that.[49]
Due to their long legs, an alternative hypothesis suggested by Björn Kurtén is that it may have hunted by running down Pleistocene herbivores such as wild horses and saiga antelopes, an idea that at one time earned it the name "running bear".[54][56][136] However, during pursuit of speedy game animals, the bear's sheer physical mass, inflexible spine and plantigrade gait would be a handicap; modern brown bears can run at the same speed but quickly tire and cannot keep up a chase for long. Correspondingly, although a 700 kg (1,500 lb) Arctodus may have been able to reach a maximum speed of 51 kilometres per hour (32 mph), all modern bears have maximum speeds significantly lower than mass-based calculations for speed. As a result, paleontologist Paul Matheus suggests that Arctodus' top speed was 40–45 km/h (25–28 mph). Arctodus skeletons do not articulate in a way that would have allowed for quick turns – an ability required of any predator that survives by chasing down agile prey.[50][61][55] Proportionally taller legs, a short trunk, proximally elongated limbs, a stride which had little to no unsupported intervals, small and laterally-orientated eyes, and proportionally short canines ill-suited for spinal and tracheal attacks further complicated ambush hunting as a lifestyle for Arctodus.[36][61][77]
Furthermore, the lack of definitive predatory adaptions (such as the absence of laterally compressed canines, and carnassials built for crushing and grinding rather than shearing meat) puts doubt to any species-wide hyper-carnivorous interpretations of A. simus.[56][81][61][75] The anatomical requirements for a large, cursorial, hyper-carnivorous bear are present in Huracan and Agriotherium, but not Arctodus.[77][258] Adaptations for predatory behavior are highly divergent in ursids versus other carnivorans, with features such as a short rostrum and long carnassials not being indicative of a predatory lifestyle in Arctodus.[75] Although the only living hyper-carnivorous ursid, the polar bear, also lacks carnassial shears, the species' specialization on small prey and reliance on blubber (rather than coarser flesh) invalidates this comparison with Arctodus.[36][61][75] However, both Arctodus simus and polar bears may have had similar overall limb proportions.[80] Regardless, carnivory was likely limited to the regular scavenging of carcasses and opportunistic hunting, as is the case with the modern brown bear.[54][56][61]
Specialist kleptoparasite vs Omnivore

The idea that Arctodus simus was an obligate kleptoparasite was most notably proposed by Paul Matheus.[50] Under this model, A. simus was ill-equipped to be an active predator, having evolved as a specialized scavenger adapted to cover an extremely large home range in order to seek out broadly and unevenly distributed mega-mammal carcasses.[61] There would have been additional selective pressure for increased body size, so that Arctodus could procure and defend carcasses from other large carnivores, some of which were gregarious, or chase them from their kills and steal their food.[55] Matheus calculated that with a hyper-carnivorous diet, a 700 kg (1,500 lb) Beringian Arctodus would need to consume ~5,853 kilograms (12,904 lb) of meat per year- the equivalent of 12 bison, 44.6 horses, or 2 woolly mammoths (adjusted for the non-edible portions of the body). Therefore, Arctodus would have had to obtain 100 kg (220 lb) of flesh/edible carrion every 6.25 days (16 kg (35.3 lb) per day).[50][76][120]
Furthermore, the short rostrum, resulting in increased out-forces of the jaw-closing muscles (temporalis and masseter), may have been an adaptation for cracking bones with their broad carnassials. Such use of the P4 and m1 teeth is supported by the heavy wear on these teeth in old individuals of Arctodus simus and Agriotherium (another giant bear).[61] Additionally, strengthened tooth enamel in Arctodus may have evolved to crack bone.[74] Moreover, at least in Beringia, the conservative growth strategies, long lives and low natural mortality rates of horses and mammoths should have provided somewhat evenly distributed carcasses throughout the year (unlike ruminants such as bison, whose mortality peaks in late winter to early spring).[76] Finally, that Arctodus and the cave hyena did not spread into Siberia and North America respectively suggests some form of competitive exclusion was at play.[70]
Rebuttal

The kleptoparasite hypothesis has been repeatedly challenged. The short, broad rostrum of Arctodus is a characteristic also shared with the sun bear and the spectacled bear, which are both omnivorous.[56] Specialized scavengers like hyenas show distinctive patterns of molar damage from cracking bones. Based on lack of "bone-cracking" wear in specimens from Rancho La Brea, researchers in 2013 concluded that A. simus was not a specialized scavenger. Of living bears, this population of A. simus showed the most similar tooth wear patterns to its closest living relative, the spectacled bear, which can have a highly varied diet ranging from omnivory to almost pure herbivory.[36][26]
Additionally, severe tooth crown fractures and alveolar infections were found in the South American giant short-faced bear (Arctotherium angustidens). These were interpreted as evidence of feeding on hard materials (e.g. bones), which could tentatively indicate for these bears the regular scavenging of ungulate carcasses obtained through kleptoparasitism. However, such dental pathologies were not observed in various specimens of A. simus, other than the strong wear facets of old individuals.[56][101] Instead, recovered dental damage (incisor wear, dental calculus & cavities) is herbivorous in origin.[62][88][21] Moreover, researchers in 2015 reviewing links between canine breakage, microwear texture patterns and carnivorans from La Brea found that A. simus consumed foods softer yet tougher than black bears and polar bears, avoided hard/brittle foods such as bone, and reaffirmed affinities between A. simus and modern, largely herbivorous spectacled bears.[103] In addition to hyenas, many other fauna did not cross the Rancholabrean Beringian gap, such as the American badger, Bootherium and the woolly rhino).[259][260][261]
Furthermore, the relative lack of Arctodus remains at predator traps such as the La Brea Tar Pits, suggests that Arctodus did not regularly compete for carcasses.[88] Although La Brea has produced more Arctodus simus specimens than any other site, Arctodus represents only 1% of all carnivorans in the pits.[103] While more abundant than brown bears and black bears, Arctodus was calculated to its baseline continental abundance, contrasting with the overabundance of other large carnivorans.[262] A similar rate (~0.9%) of relative abundance was calculated for Arctodus compared to other megafauna at the Natural Trap Cave in Wyoming by 1993.[263] Additionally, isotope analyses of Beringian Arctodus specimens suggest that Arctodus had a low consumption rate of horses and mammoths in Beringia, despite those species making up ~50% of the available biomass in Beringia.[108] Further evidence comes from the evolution of brain size relative to body size- bears with high caloric diets and which do not exhibit dormancy showed a weak but significant correlation with bigger relative brain size. Arctodus simus plotted in between the likely hypercarnivorous Cephalogale, and the almost exclusively herbivorous Eurasian cave bear and Indarctos, suggesting omnivory.[264]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Arctodus pristinus" (in en-US). 2017-03-30. https://www.floridamuseum.ufl.edu/florida-vertebrate-fossils/species/arctodus-pristinus/.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 Emslie, Steven D. (1995). "The fossil record of Arctodus pristinus (Ursidae: Tremarctinae) in Florida". Bulletin of the Florida Museum of Natural History 37 (15): 501–514. doi:10.58782/flmnh.hduf9651. https://www.floridamuseum.ufl.edu/wp-content/uploads/sites/35/2017/03/Vol-37-No-15.pdf.
- ↑ "South Carolina Fossils" (in en). Nature 20 (510): 354–355. 1879-08-01. doi:10.1038/020354a0. ISSN 1476-4687. Bibcode: 1879Natur..20..354..
- ↑ 4.0 4.1 Cope E. D. (1879). "The cave bear of California". American Naturalist 13: 791.
- ↑ 5.0 5.1 5.2 Feranec, Robert S. (November 2009). "Implications of Radiocarbon Dates from Potter Creek Cave, Shasta County, California, USA". Radiocarbon 51 (3): 931–936. doi:10.1017/S0033822200034007. Bibcode: 2009Radcb..51..931F.
- ↑ 6.0 6.1 Merriam, John C.; Stock, Chester (1925) (in en), Relationships and Structure of the Short-Faced Bear, Arctotherium, from the Pleistocene of California, Washington, DC: Carnegie institution of Washington, pp. 1–25, https://resolver.caltech.edu/CaltechAUTHORS:20191008-132308406, retrieved 2022-05-06
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 7.20 7.21 7.22 7.23 7.24 7.25 7.26 7.27 7.28 Richards, Ronald L.; Churcher, C.S.; Turnbull, William D. (1996-12-31), "Distribution and size variation in North American Short-faced bears, Arctodus simus", Palaeoecology and Palaeoenvironments of Late Cenozoic Mammals (University of Toronto Press): pp. 191–246, doi:10.3138/9781487574154-012, ISBN 978-1-4875-7415-4, https://www.degruyter.com/document/doi/10.3138/9781487574154-012/html, retrieved 2023-11-15
- ↑ 8.0 8.1 8.2 Sanders, Albert E. (2002). Additions to the Pleistocene Mammal Faunas of South Carolina, North Carolina, and Georgia. American Philosophical Society. ISBN 978-0-87169-925-1. https://books.google.com/books?id=LS8LAAAAIAAJ&dq=pristinus+south+carolina&pg=PA48.
- ↑ "Arctodus". https://www.utep.edu/leb/pleistnm/taxaMamm/Arctodus.htm#:~:text=Synonyms.,Arctotherium%20simum,%20Tremarctotherium%20simum.
- ↑ Spamer, Earle E.; Daeschler, Edward; Philadelphia, Academy of Natural Sciences of; Vostreys-Shapiro, L. Gay (1995) (in en). A Study of Fossil Vertebrate Types in the Academy of Natural Sciences of Philadelphia: Taxonomic, Systematic, and Historical Perspectives. Academy of Natural Sciences. ISBN 978-0-910006-51-4. https://books.google.com/books?id=3loD7lTLBxgC&dq=Arctodus+haplodon&pg=PA204.
- ↑ Hay, Oliver Perry (1901) (in en). Bibliography and Catalogue of the Fossil Vertebrata of North America. U.S. Government Printing Office. https://books.google.com/books?id=mkMZAAAAYAAJ&q=haplodon&pg=PA763.
- ↑ Kurtén, Björn (June 1, 1963). "Fossil Bears from Texas". The Pearce-Sellards Series 1. https://repositories.lib.utexas.edu/bitstream/handle/2152/29869/tmm-pss-01.pdf?sequence=1.
- ↑ 13.0 13.1 Gidley, James W.; Gazin, Charles Lewis (1938). "The Pleistocene vertebrate fauna from Cumberland Cave, Maryland" (in en). Smithsonian Institution United States National Museum Bulletin 171. ISSN 0362-9236. http://hdl.handle.net/10088/10106.
- ↑ 14.0 14.1 Ferrusquía-Villafranca, Ismael; Arroyo-Cabrales, Joaquín; Martínez-Hernández, Enrique; Gama-Castro, Jorge; Ruiz-González, José; Polaco, Oscar J.; Johnson, Eileen (2010-04-15). "Pleistocene mammals of Mexico: A critical review of regional chronofaunas, climate change response and biogeographic provinciality" (in en). Quaternary International. Faunal Dynamics and Extinction in the Quaternary: Studies in Honor of Ernest L. Lundelius, Jr. 217 (1): 53–104. doi:10.1016/j.quaint.2009.11.036. ISSN 1040-6182. Bibcode: 2010QuInt.217...53F. https://www.sciencedirect.com/science/article/pii/S104061820900442X.
- ↑ Arroyo-Cabrales, Joaquín; Polaco, Oscar J.; Johnson, Eileen; Ferrusquía-Villafranca, Ismael (2010-02-01). "A perspective on mammal biodiversity and zoogeography in the Late Pleistocene of México" (in en). Quaternary International. Quaternary Changes of Mammalian Communities Across and Between Continents 212 (2): 187–197. doi:10.1016/j.quaint.2009.05.012. ISSN 1040-6182. Bibcode: 2010QuInt.212..187A. https://www.sciencedirect.com/science/article/pii/S1040618209001633.
- ↑ Schubert, Blaine W.; Chatters, James C.; Arroyo-Cabrales, Joaquin; Samuels, Joshua X.; Soibelzon, Leopoldo H.; Prevosti, Francisco J.; Widga, Christopher; Nava, Alberto et al. (May 2019). "Yucatán carnivorans shed light on the Great American Biotic Interchange". Biology Letters 15 (5). doi:10.1098/rsbl.2019.0148. PMID 31039726.
- ↑ 17.0 17.1 17.2 McLellan, Bruce; Reiner, David C. (1994). "A Review of bear evolution". Int. Conf. Bear Res. And Manage 9 (1): 85–96. doi:10.2307/3872687. http://www.bearbiology.com/fileadmin/tpl/Downloads/URSUS/Vol_9/McLellan_Reiner_Vol_9.pdf.
- ↑ "Big Pine Citizen 2 March 1918 — California Digital Newspaper Collection". https://cdnc.ucr.edu/?a=d&d=BPC19180302.2.28.
- ↑ 19.00 19.01 19.02 19.03 19.04 19.05 19.06 19.07 19.08 19.09 Pedersen, Mikkel Winther; De Sanctis, Bianca; Saremi, Nedda F.; Sikora, Martin; Puckett, Emily E.; Gu, Zhenquan; Moon, Katherine L.; Kapp, Joshua D. et al. (2021-06-21). "Environmental genomics of Late Pleistocene black bears and giant short-faced bears". Current Biology 31 (12): 2728–2736.e8. doi:10.1016/j.cub.2021.04.027. PMID 33878301. Bibcode: 2021CBio...31E2728P.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 Saremi, Nedda F. (September 2020). "A Genomic Approach to Studying the Evolutionary Consequences of Population Declines, Chapter 2: Tremarctine bears of present and past". Biomolecular Engineering and Bioinformatics, University of Santa Cruz (PHD Thesis): 86–142. https://escholarship.org/content/qt8f63q6wg/qt8f63q6wg_noSplash_1cb133996a69d42cb301dce3d6461d91.pdf?t=qiwqjp.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 21.12 21.13 21.14 21.15 21.16 21.17 21.18 21.19 21.20 21.21 21.22 21.23 21.24 21.25 21.26 Steffen, Martina L.; Fulton, Tara L. (2018-02-01). "On the association of giant short-faced bear (Arctodus simus) and brown bear (Ursus arctos) in late Pleistocene North America". Geobios 51 (1): 61–74. doi:10.1016/j.geobios.2017.12.001. Bibcode: 2018Geobi..51...61S.
- ↑ Soibelzon, Leopoldo H.; Romero, M.R. Aguilar (2008-10-14). "A Blancan (Pliocene) short-faced bear from El Salvador and its implications for Tremarctines in South America". Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen 250 (1): 1–8. doi:10.1127/0077-7749/2008/0250-0001. Bibcode: 2008NJGPA.250....1S. http://sedici.unlp.edu.ar/handle/10915/5361.
- ↑ 23.00 23.01 23.02 23.03 23.04 23.05 23.06 23.07 23.08 23.09 23.10 23.11 23.12 23.13 23.14 23.15 23.16 23.17 Schubert, Blaine; Hulbert, Richard; MacFadden, Bruce; Searle, Michael; Searle, Seina (2010-01-01). "Giant Short-faced Bears (Arctodus simus) in Pleistocene Florida USA, a Substantial Range Extension". Journal of Paleontology 84 (1): 79–87. doi:10.1666/09-113.1. Bibcode: 2010JPal...84...79S. https://www.researchgate.net/publication/250071137.
- ↑ 24.0 24.1 24.2 24.3 Mitchell, Kieren J.; Bray, Sarah C.; Bover, Pere; Soibelzon, Leopoldo; Schubert, Blaine W.; Prevosti, Francisco; Prieto, Alfredo; Martin, Fabiana et al. (2016-04-30). "Ancient mitochondrial DNA reveals convergent evolution of giant short-faced bears (Tremarctinae) in North and South America". Biology Letters 12 (4). doi:10.1098/rsbl.2016.0062. PMID 27095265.
- ↑ Krause, Johannes; Unger, Tina; Noçon, Aline; Malaspinas, Anna-Sapfo; Kolokotronis, Sergios-Orestis; Stiller, Mathias; Soibelzon, Leopoldo; Spriggs, Helen et al. (December 2008). "Mitochondrial genomes reveal an explosive radiation of extinct and extant bears near the Miocene-Pliocene boundary" (in en). BMC Evolutionary Biology 8 (1): 220. doi:10.1186/1471-2148-8-220. ISSN 1471-2148. PMID 18662376. Bibcode: 2008BMCEE...8..220K.
- ↑ 26.00 26.01 26.02 26.03 26.04 26.05 26.06 26.07 26.08 26.09 Donohue, Shelly L.; DeSantis, Larisa R. G.; Schubert, Blaine W.; Ungar, Peter S. (30 October 2013). "Was the giant short-faced bear a hyper-scavenger? A new approach to the dietary study of ursids using dental microwear textures". PLOS ONE 8 (10). doi:10.1371/journal.pone.0077531. PMID 24204860. Bibcode: 2013PLoSO...877531D.
- ↑ Morgan, Gary S. (2005). "The Great American Biotic Interchange in Florida". Bulletin of the Florida Museum of Natural History 45 (4): 271–311. doi:10.58782/flmnh.pkqn7297. https://www.floridamuseum.ufl.edu/wp-content/uploads/sites/35/2017/03/bulletin-Morganlowres.pdf.
- ↑ Hulbert, Richard (2010-09-20). "A new early Pleistocene tapir (Mammalia: Perissodactyla) from Florida, with a review of Blancan tapirs from the state" (in en). Bulletin of the Florida Museum of Natural History 49 (3): 67–126. doi:10.58782/flmnh.ezjr9001. ISSN 2373-9991. https://flmnhbulletin.com/index.php/flmnh/article/view/flmnh-vol49-no3.
- ↑ 29.0 29.1 White Jr., Richard S.; Morgan, Gary S. (2005). "Arizonan Blancan Vertebrate Faunas in Regional Perspective". Vertebrate Paleontology of Arizona, Mesa Southwest Museum Bulletin 11. https://www.researchgate.net/publication/267844516.
- ↑ 30.0 30.1 Thrasher, Larry (July 2022). "Fossils and Age Relationships of the Late Pliocene and Early Pleistocene (Blancan) 111 Ranch Beds and Bear Springs Wash Beds, Graham County, Arizona". Late Cenozoic Vertebrate Paleontology: Tribute to Arthur H. Harris. New Mexico Museum of Natural History and Science Bulletin 88. https://www.researchgate.net/publication/362191500.
- ↑ Morgan, Gary S.; White Jr., Richard S. (2005). "Miocene and Pliocene vertebrates from Arizona". Vertebrate Paleontology in Arizona. New Mexico Museum of Natural History and Science Bulletin 29. https://www.researchgate.net/publication/40661398.
- ↑ Morgan, Gary S.; Lucaas, Spencer G. (October 12–14, 2001). "Plioi-Pleistocene Stratigraphy and Geomorphology of the Central Part of the Albuquerque Basin". D1-D4 (B29-B30). https://geoinfo.nmt.edu/publications/openfile/downloads/400-499/454/papers/OFR454D_PDF/H_OFR454D_Morgan_PlioceneBiostratigraphy_reprint.pdf. Retrieved June 17, 2025.
- ↑ Brown, Gary (1996). Great Bear Almanac. Lyons & Burford. p. 340. ISBN 978-1-55821-474-3. https://archive.org/details/greatbearalmanac00gary/page/340.
- ↑ 34.0 34.1 Russell, Dale A.; Rich, Fredrick J.; Schneider, Vincent; Lynch-Stieglitz, Jean (May 2009). "A warm thermal enclave in the Late Pleistocene of the Southeastern United States". Biological Reviews 84 (2): 173–202. doi:10.1111/j.1469-185X.2008.00069.x. PMID 19391200.
- ↑ 35.0 35.1 35.2 35.3 Bell, Christopher; Lundelius, Ernest L.; Barnosky, Anthony D.; Zarzewski, Richard J.; Graham, Russell; Lindsay, Everett H.; Ruez, Dennis R.; Semken, Holmes A. et al. (2004-04-21). "Chapter 7: The Blancan, Irvingtonian, and Rancholabrean Mammal Ages" (in en). Late Cretaceous and Cenozoic Mammals of North America. Columbia University Press. doi:10.7312/wood13040. ISBN 978-0-231-50378-5. https://www.researchgate.net/publication/263425514.
- ↑ 36.00 36.01 36.02 36.03 36.04 36.05 36.06 36.07 36.08 36.09 36.10 36.11 36.12 36.13 36.14 36.15 36.16 Emslie, Steven D.; Czaplewski, Nicholas J. (1985-11-15). "A new record of giant short-faced bear, Arctodus simus, from western North America with a re-evaluation of its paleobiology". Contributions in Science 371: 1–12. doi:10.5962/p.226835. https://www.biodiversitylibrary.org/partpdf/226835.
- ↑ 37.0 37.1 37.2 Martin, Larry; Neuner, A. (1978-01-01). "The End of the Pleistocene in North America". Transactions of the Nebraska Academy of Sciences and Affiliated Societies. https://digitalcommons.unl.edu/tnas/337.
- ↑ 38.0 38.1 38.2 38.3 38.4 38.5 38.6 Trayler, Robin B.; Dundas, Robert G.; Fox-Dobbs, Kena; Van De Water, Peter K. (2015-11-01). "Inland California during the Pleistocene—Megafaunal stable isotope records reveal new paleoecological and paleoenvironmental insights" (in en). Palaeogeography, Palaeoclimatology, Palaeoecology 437: 132–140. doi:10.1016/j.palaeo.2015.07.034. ISSN 0031-0182. Bibcode: 2015PPP...437..132T. https://www.sciencedirect.com/science/article/pii/S0031018215004010.
- ↑ 39.0 39.1 39.2 39.3 39.4 39.5 39.6 Pérez-Crespo, Víctor Adrián; Arroyo-Cabrales, Joaquín; Morales-Puente, Pedro; Cienfuegos-Alvarado, Edith; Otero, Francisco J. (March 2018). "Diet and habitat of mesomammals and megamammals from Cedral, San Luis Potosí, México". Geological Magazine 155 (3): 674–684. doi:10.1017/S0016756816000935. Bibcode: 2018GeoM..155..674P.
- ↑ 40.0 40.1 Harris, Arthur H. (November 1985). "Preliminary report on the vertebrate fauna of U-Bar Gave, Hidalgo County, New Mexico". New Mexico Geology 7 (4): 74–84. doi:10.58799/NMG-v7n4.74. https://geoinfo.nmt.edu/publications/periodicals/nmg/7/n4/nmg_v7_n4_p74.pdf.
- ↑ 41.00 41.01 41.02 41.03 41.04 41.05 41.06 41.07 41.08 41.09 41.10 Esker, Donald; Wilkins, William; Agenbroad, Larry (2010-08-13). "Esker, Wilkins, and Agenbroad—Multivariate Analysis Of Ursids: A multivariate analysis of the ecology of North American Pleistocene bears, with a focus on Arctodus simus". https://www.researchgate.net/publication/314037201.
- ↑ Akersten, William A. (1996-12-31), "Diversity bottlenecks, oddball survivors, and negative keys", Palaeoecology and Palaeoenvironments of Late Cenozoic Mammals (University of Toronto Press): pp. 1–15, doi:10.3138/9781487574154-004, ISBN 978-1-4875-7415-4, https://www.degruyter.com/document/doi/10.3138/9781487574154-004/html, retrieved 2024-01-23
- ↑ Phillips, George Edward (6 August 2006). "Paleofaunistics of Nonmammalian Vertebrates from the Late Pleistocene of the Mississippi-Alabama Black Prairie". North Carolina State University (Masters). https://repository.lib.ncsu.edu/items/185efe46-230f-4ff1-87be-12da682be6e8.
- ↑ 44.0 44.1 44.2 44.3 44.4 44.5 44.6 Scott, Eric; Cox, Shelley M. (May 24, 1993). "Arctodus simus (Cope, 1879) from Riverside County, California". PaleoBios 15 (2): 27–36. https://ucmp.berkeley.edu/science/paleobios/backissues/v15no2_scott&cox.pdf.
- ↑ 45.0 45.1 45.2 Hill, Christopher L.; Wilson, Mike C. (2000). "The Doeden Local Fauna (Illinoian/Sangamonian?), Eastern Montana". Unknown: 140–142. https://www.researchgate.net/publication/270216333.
- ↑ 46.0 46.1 46.2 46.3 46.4 46.5 46.6 Kurtén, Björn (1967) (in en). Pleistocene Bears of North America: Genus Arctodus, short-faced bears. Societas pro Fauna et Flora Fennica. https://books.google.com/books?id=GkCFtAEACAAJ.
- ↑ 47.0 47.1 47.2 Van Valkenburgh, Blaire; Hayward, Matthew W.; Ripple, William J.; Meloro, Carlo; Roth, V. Louise (2016-01-26). "The impact of large terrestrial carnivores on Pleistocene ecosystems" (in en). Proceedings of the National Academy of Sciences 113 (4): 862–867. doi:10.1073/pnas.1502554112. ISSN 0027-8424. PMID 26504224. Bibcode: 2016PNAS..113..862V.
- ↑ 48.0 48.1 48.2 Fowler, Nicholas L.; Spady, Thomas J.; Wang, Guiming; Leopold, Bruce D.; Belant, Jerrold L. (October 2021). "Denning, metabolic suppression, and the realisation of ecological opportunities in Ursidae". Mammal Review 51 (4): 465–481. doi:10.1111/mam.12246. Bibcode: 2021MamRv..51..465F.
- ↑ 49.0 49.1 49.2 49.3 Soibelzon, Leopoldo H.; Schubert, Blaine W. (2011). "The Largest Known Bear, Arctotherium Angustidens, from the Early Pleistocene Pampean Region of Argentina: With a Discussion of Size and Diet Trends in Bears". Journal of Paleontology 85 (1): 69–75. doi:10.1666/10-037.1.
- ↑ 50.0 50.1 50.2 50.3 50.4 Nancy Sisinyak. "The Biggest Bear ... Ever". Alaska Fish and Wildlife News. https://www.adfg.alaska.gov/index.cfm?adfg=wildlifenews.view_article&articles_id=232.
- ↑ 51.0 51.1 Fisher, W. A. (2018-02-05). "How Big Was This Short-Faced Bear?" (in en). https://bear.org/how-big-was-this-short-faced-bear/.
- ↑ "Giant Short-faced Bear | Yukon Beringia Interpretive Centre". https://www.beringia.com/exhibit/ice-age-animals/giant-short-faced-bear.
- ↑ 53.0 53.1 53.2 53.3 53.4 Richards, Ronald L.; Neiburger, Ellis J.; Turnbull, William D. (1995). Giant short-faced bear (Arctodus simus yukonensis) remains from Fulton County, northern Indiana. Chicago, Ill.: Field Museum of Natural History. https://www.biodiversitylibrary.org/bibliography/3480.
- ↑ 54.0 54.1 54.2 54.3 54.4 54.5 54.6 54.7 Mattson, David J. (1998). "Diet and Morphology of Extant and Recently Extinct Northern Bears". Ursus 10: 479–496.
- ↑ 55.0 55.1 55.2 55.3 55.4 55.5 55.6 55.7 55.8 Matheus, Paul E. (2003). Locomotor adaptations and ecomorphology of short-faced bears (Arctodus simus) in eastern Beringia. Yukon Palaeontologist, Gov't. of Yukon. OCLC 243520303.
- ↑ 56.00 56.01 56.02 56.03 56.04 56.05 56.06 56.07 56.08 56.09 56.10 56.11 56.12 56.13 56.14 56.15 56.16 56.17 56.18 Figueirido (2010). "Demythologizing Arctodus simus, the 'short-faced' long-legged and predaceous bear that never was". Journal of Vertebrate Paleontology 30 (1): 262–275. doi:10.1080/02724630903416027. Bibcode: 2010JVPal..30..262F.
- ↑ 57.0 57.1 57.2 57.3 57.4 57.5 Schubert, Blaine W. (2010-04-15). "Late Quaternary chronology and extinction of North American giant short-faced bears (Arctodus simus)". Quaternary International. Faunal Dynamics and Extinction in the Quaternary: Studies in Honor of Ernest L. Lundelius, Jr. 217 (1): 188–194. doi:10.1016/j.quaint.2009.11.010. Bibcode: 2010QuInt.217..188S.
- ↑ 58.00 58.01 58.02 58.03 58.04 58.05 58.06 58.07 58.08 58.09 Salis, A. T.; Schubert, B. W.; Bray, S. C. E.; Heiniger, H.; Meachen, J.; Cooper, A.; Mitchell, K. J. (2025). "Genetic diversity, phylogeography, and sexual dimorphism in the extinct giant short-faced bear (Arctodus simus)". Zoological Journal of the Linnean Society 203 (2): zlaf001. doi:10.1093/zoolinnean/zlaf001.
- ↑ 59.0 59.1 Christiansen, Per (1999). "What size were Arctodus simus and Ursus spelaeus (Carnivora: Ursidae)?". Annales Zoologici Fennici 36 (2): 93–102.
- ↑ 60.0 60.1 60.2 60.3 Voorhies, M. R.; Corner, R. G. (1986). "Giant Bear Arctodus as a Potential Breaker and Flaker of Late Pleistocene Megafaunal Remains". Current Research in the Pleistocene 3: 49–51. https://liberalarts.tamu.edu/csfa/wp-content/uploads/sites/14/2023/07/CRP-3-1986.pdf.
- ↑ 61.00 61.01 61.02 61.03 61.04 61.05 61.06 61.07 61.08 61.09 61.10 61.11 61.12 61.13 61.14 61.15 61.16 61.17 Sorkin, B. (January 2006). "Ecomorphology of the giant short-faced bears Agriotherium and Arctodus". Historical Biology 18 (1): 1–20. doi:10.1080/08912960500476366. Bibcode: 2006HBio...18....1S.
- ↑ 62.0 62.1 62.2 62.3 62.4 62.5 62.6 62.7 62.8 Schubert, Blaine; Kaufmann, James (2003-08-01). "A partial short-faced bear skeleton from an Ozark Cave with comments on the paleobiology of the species". Journal of Cave and Karst Studies 65. https://www.researchgate.net/publication/252748398.
- ↑ 63.0 63.1 63.2 63.3 63.4 Schubert, Blaine W.; Wallace, Steven C. (August 2009). "Late Pleistocene giant short-faced bears, mammoths, and large carcass scavenging in the Saltville Valley of Virginia, USA" (in en). Boreas 38 (3): 482–492. doi:10.1111/j.1502-3885.2009.00090.x. Bibcode: 2009Borea..38..482S. https://onlinelibrary.wiley.com/doi/10.1111/j.1502-3885.2009.00090.x.
- ↑ 64.0 64.1 64.2 64.3 64.4 64.5 Stark, Mike (2022). Chasing the Ghost Bear: On the Trail of America's Lost Super Beast. University of Nebraska Press. doi:10.2307/j.ctv2br108d. ISBN 978-1-4962-2902-1.
- ↑ Goswami, Anjali; Milne, Nick; Wroe, Stephen (2011-06-22). "Biting through constraints: cranial morphology, disparity and convergence across living and fossil carnivorous mammals" (in en). Proceedings of the Royal Society B: Biological Sciences 278 (1713): 1831–1839. doi:10.1098/rspb.2010.2031. ISSN 0962-8452. PMID 21106595.
- ↑ 66.0 66.1 66.2 Matheus, Paul E. (1995-11-01). "Diet and Co-ecology of Pleistocene Short-Faced Bears and Brown Bears in Eastern Beringia" (in en). Quaternary Research 44 (3): 447–453. doi:10.1006/qres.1995.1090. ISSN 0033-5894. Bibcode: 1995QuRes..44..447M. https://www.sciencedirect.com/science/article/pii/S0033589485710903.
- ↑ 67.0 67.1 67.2 67.3 67.4 67.5 67.6 Figueirido, Borja; Soibelzon, Leopoldo H. (2009-08-19). "Inferring palaeoecology in extinct tremarctine bears (Carnivora, Ursidae) using geometric morphometrics". Lethaia 43 (2): 209–222. doi:10.1111/j.1502-3931.2009.00184.x. http://sedici.unlp.edu.ar/handle/10915/5372.
- ↑ 68.0 68.1 68.2 68.3 Gillette, David D.; Madsen, David B. (1992-03-06). "The short-faced bear Arctodus simus from the late Quaternary in the Wasatch Mountains of central Utah". Journal of Vertebrate Paleontology 12 (1): 107–112. doi:10.1080/02724634.1992.10011436. Bibcode: 1992JVPal..12..107G.
- ↑ 69.0 69.1 69.2 Slaughter, B. H.; Crook, W. W. Jr.; Harris, R. K.; Allen, D. C.; Seifert, M. (1962-01-01). The Hill-Shuler Local Faunas of the Upper Trinity River, Dallas and Denton Counties, Texas. Report Investigation. The University of Texas at Austin, Bureau of Economic Geology. doi:10.23867/ri0048d.
- ↑ 70.0 70.1 70.2 70.3 Baryshnikov, Gennady (1994). Agenbroad, Larry D.. ed. The Hot Springs Mammoth Site: A Decade of Field and Laboratory Research in Paleontology, Geology and Paleoecology. The Mammoth Site of Hot Springs, South Dakota Inc.. pp. Chapter 16. https://www.researchgate.net/publication/336916777.
- ↑ Dickinson, Edwin; Elminowski, Erin E.; Flores, Deanna; Eldridge, Emma I.; Granatosky, Michael C.; Hartstone-Rose, Adam (October 2022). "A morphological analysis of carnivoran ossicles from Rancho La Brea" (in en). Journal of Morphology 283 (10): 1337–1349. doi:10.1002/jmor.21506. ISSN 0362-2525. PMID 36041006. Bibcode: 2022JMorp.283.1337D.
- ↑ Arnaudo, Maria Eugenia; Bona, Paula; Soibelzon, Leopoldo Hector; Schubert, Blaine W. (December 2016). "Anatomical study of the auditory region of Arctotherium tarijense (Ursidae, Tremarctinae), an extinct short-faced bear from the Pleistocene of South America". Journal of Anatomy 229 (6): 825–837. doi:10.1111/joa.12525. ISSN 0021-8782. PMID 27460048.
- ↑ 73.0 73.1 Meloro, Carlo (2011-03-17). "Feeding habits of Plio-Pleistocene large carnivores as revealed by the mandibular geometry" (in en). Journal of Vertebrate Paleontology 31 (2): 428–446. doi:10.1080/02724634.2011.550357. ISSN 0272-4634. Bibcode: 2011JVPal..31..428M. https://www.tandfonline.com/doi/full/10.1080/02724634.2011.550357.
- ↑ 74.0 74.1 Stefen, Clara (2001). "Enamel Structure of Arctoid Carnivora: Amphicyonidae, Ursidae, Procyonidae, and Mustelidae". Journal of Mammalogy 82 (2): 450–462. doi:10.1644/1545-1542(2001)082<0450:ESOACA>2.0.CO;2. ISSN 0022-2372.
- ↑ 75.0 75.1 75.2 75.3 75.4 75.5 Figueirido, B.; Palmqvist, P.; Pérez-Claros, J. A. (2008-12-22). "Ecomorphological correlates of craniodental variation in bears and paleobiological implications for extinct taxa: an approach based on geometric morphometrics" (in en). Journal of Zoology 277 (1): 70–80. doi:10.1111/j.1469-7998.2008.00511.x. ISSN 0952-8369. https://zslpublications.onlinelibrary.wiley.com/doi/10.1111/j.1469-7998.2008.00511.x.
- ↑ 76.0 76.1 76.2 76.3 76.4 76.5 76.6 Matheus, Paul Edward (1997). Paleoecology and ecomorphology of the giant short-faced bear in Eastern Beringia (PhD thesis).
- ↑ 77.0 77.1 77.2 77.3 77.4 77.5 77.6 Jiangzuo, Qigao; Flynn, John J.; Wang, Shiqi; Hou, Sukuan; Deng, Tao (2023-03-14). "New Fossil Giant Panda Relatives (Ailuropodinae, Ursidae): A Basal Lineage of Gigantic Mio-Pliocene Cursorial Carnivores". American Museum Novitates (3996): 1–71. doi:10.1206/3996.1. ISSN 0003-0082.
- ↑ 78.0 78.1 Churcher, C. S.; Morgan, A. V.; Carter, L. D. (2011-02-08). "Arctodus simus from the Alaskan Arctic Slope" (in en). Canadian Journal of Earth Sciences 30 (5): 1007–1013. doi:10.1139/e93-084. https://cdnsciencepub.com/doi/10.1139/e93-084.
- ↑ 79.0 79.1 79.2 Lynch, Eric Randally (2012-08-06). Cursorial Adaptations in the Forelimb of the Giant Short-Faced Bear, Arctodus simus, Revealed by Traditional and 3D Landmark Morphometrics. East Tennessee State University. OCLC 818344518.
- ↑ 80.0 80.1 80.2 Samuels, Joshua X.; Meachen, Julie A.; Sakai, Stacey A. (13 September 2012). "Postcranial morphology and the locomotor habits of living and extinct carnivorans" (in en). Journal of Morphology 274 (2): 121–146. doi:10.1002/jmor.20077. ISSN 0362-2525. PMID 22972188. https://onlinelibrary.wiley.com/doi/10.1002/jmor.20077.
- ↑ 81.0 81.1 81.2 Meloro, Carlo; de Oliveira, Alessandro Marques (2019-03-01). "Elbow Joint Geometry in Bears (Ursidae, Carnivora): a Tool to Infer Paleobiology and Functional Adaptations of Quaternary Fossils". Journal of Mammalian Evolution 26 (1): 133–146. doi:10.1007/s10914-017-9413-x. https://researchonline.ljmu.ac.uk/id/eprint/7536/1/Meloro_et_al-2017-Journal_of_Mammalian_Evolution.pdf.
- ↑ Packard, E.L.; Allison, I.S.; Cressman, L.S.. "Mammalian Tracks in the Late Pliocene or Early Pleistocene Beds of Lake County Oregon". https://www.oregongeology.org/milo/archive/MiningDistricts/LakeCounty/UnclassifiedDistrict/LakeCountyFossilLocalities/LakeCountyFossilLocalitiesReports.pdf.
- ↑ Gentry, Andrew; Franco, Christopher Michael; Bustos, David (September 2011). "Mammalian Ichnofauna from the Upper-Pleistocene deposits of White Sands National Monument, Otero County, New Mexico.". Geoscientists-in-the Parks, National Park Service, Denver, Colorado.. https://www.researchgate.net/publication/325261275.
- ↑ Nadeau, Andy J.; Allen, Kathy; Benck, Kevin; Davis, Anna M.; Hutchins, Hannah; Gardner, Sarah; Amberg, Shannon; Robertson, Andrew (September 2017). "White Sands National Monument Natural Resource Condition Assessment". Natural Resource Report NPS/WHSA/NRR—2017/1508: 276–277. http://npshistory.com/publications/whsa/nrr-2017-1508.pdf.
- ↑ 85.0 85.1 Lucas, Spencer G.; Spielmann, Justin A.; Lockley, Martin G. (2007). "Cenozoic Vertebrate Tracks and Traces" (in en). New Mexico Museum of Natural History and Science Bulletin 42. https://books.google.com/books?id=bGbmCQAAQBAJ&dq=riverbluff+cave+arctodus&pg=PA3.
- ↑ Rovey II, Charles W.; Forir, Matt; Balco, Greg; Gaunt, David (2010-01-01). "Geomorphology and Paleontology of Riverbluff Cave, Springfield, Missouri". in Evans, Kevin R. (in en). From Precambrian Rift Volcanoes to the Mississippian Shelf Margin: Geological Field Excursions in the Ozark Mountains (Field Guide 17 ed.). Geological Society of America. pp. 31–32. ISBN 978-0-8137-0017-5. https://books.google.com/books?id=HRlx-0ydi4YC&dq=riverbluff+cave&pg=PA31.
- ↑ 87.0 87.1 Salesa, M. J.; Siliceo, G.; Antón, M.; Abella, J.; Montoya, P.; Morales, J. (2006-12-30). "Anatomy of the "false thumb" of Tremarctos ornatus (Carnivora, Ursidae, Tremarctinae): Phylogenetic and functional implications". Estudios Geológicos 62 (1): 389–394. doi:10.3989/egeol.0662133. ISSN 1988-3250. http://estudiosgeol.revistas.csic.es/index.php/estudiosgeol/article/view/33/32.
- ↑ 88.00 88.01 88.02 88.03 88.04 88.05 88.06 88.07 88.08 88.09 88.10 88.11 88.12 Figueirido, Borja; Perez, Alejandro; Schubert, Blaine; Serrano, Francisco; Farrell, Aisling; Pastor, Francisco; Neves, Aline; Romero, Alejandro (2017-12-19). "Dental caries in the fossil record: A window to the evolution of dietary plasticity in an extinct bear". Scientific Reports 7 (1): 17813. doi:10.1038/s41598-017-18116-0. PMID 29259277. PMC 5736623. Bibcode: 2017NatSR...717813F. https://www.researchgate.net/publication/321791811.
- ↑ Rothschild, Bruce M.; Martin, Larry D. (2006) (in en). Skeletal Impact of Disease: Bulletin 33. New Mexico Museum of Natural History and Science. https://books.google.com/books?id=AGfmCQAAQBAJ&dq=%22arctodus%22+treponemal+infection+rothschild&pg=PA105.
- ↑ Rothschild, Bruce M. (13 October 1988). "Scientific Correspondence". Nature 335 (Existence of syphilis in a Pleistocene bear): 595. doi:10.1038/335595a0. PMID 3050529. https://www.nature.com/articles/335595a0.pdf?origin=ppub.
- ↑ Pinto, A. C.; Etxebarría, F. (2001). "Description of pathological conditions in the skeleton of an adult male brown bear Ursus arctos from the Cantabrian range of mountains (Reserva Nacional de Caza de Riaño, León) Coruña". Cadernos Lab. Xeolóxico de Laxe 2001: 471. ISSN 0213-4497. https://www.udc.es/files/iux/almacen/articulos/cd26_art33.pdf.
- ↑ 92.0 92.1 92.2 92.3 92.4 92.5 92.6 Steffen, Martina L.; Harington, C. R. Harington (2010-07-23). "Giant short-faced bear (Arctodus simus) from late Wisconsinan deposits at Cowichan Head, Vancouver Island, British Columbia" (in en). Canadian Journal of Earth Sciences 47 (8): 1029–1036. doi:10.1139/E10-018. Bibcode: 2010CaJES..47.1029S. https://cdnsciencepub.com/doi/10.1139/E10-018.
- ↑ 93.0 93.1 93.2 93.3 93.4 93.5 93.6 Bray, Sarah C. E. (September 2010). Mitochondrial DNA Analysis of the Evolution and Genetic Diversity of Ancient and Extinct Bears (PDF) (Thesis). School of Environmental and Earth Sciences, University of Adelaide. pp. 214 (230).
- ↑ Mychajliw, Alexis M.; Rick, Torben C.; Dagtas, Nihan D.; Erlandson, Jon M.; Culleton, Brendan J.; Kennett, Douglas J.; Buckley, Michael; Hofman, Courtney A. (2020-09-16). "Biogeographic problem-solving reveals the Late Pleistocene translocation of a short-faced bear to the California Channel Islands". Scientific Reports 10 (1): 15172. doi:10.1038/s41598-020-71572-z. PMID 32938967.
- ↑ 95.0 95.1 Daeschler, Edward B.; Spamer, Earl E.; Parris, David C. (1993). "Review and New Data on the Port Kennedy Local Fauna and Flora (Late Irvingtonian), Valley Forge National Historical Park, Montgomery County, Pennsylvania". The Mosasaur - Delaware Valley Paleontological Society 5: 23–41. https://www.researchgate.net/publication/325630951.
- ↑ 96.0 96.1 Eshelman, Ralph E.; Bell, Christopher; Graham, Russel W.; Semken Jr., Holmes A.; Withnell, Charles B.; Scarpetta, Simon G.; James, Helen F.; Godfrey, Stephen J. et al. (2025-04-18) (in en). Middle Pleistocene Cumberland Bone Cave Local Fauna, Allegany County, Maryland: A Systematic Revision and Paleoecological Interpretation of the Irvingtonian, Middle Appalachians, USA. Smithsonian Contributions to Paleobiology. 108. Smithsonian Institution Scholarly Press. doi:10.5479/si.28597193. https://smithsonian.figshare.com/articles/book/Middle_Pleistocene_Cumberland_Bone_Cave_Local_Fauna_Allegany_County_Maryland_A_Systematic_Revision_and_Paleoecological_Interpretation_of_the_Irvingtonian_Middle_Appalachians_USA/28597193/1.
- ↑ 97.0 97.1 97.2 97.3 97.4 97.5 Lucas, Spencer G.; Sullivan, Robert M. (in en). Vertebrate Paleontology in New Mexico: Bulletin 68. New Mexico Museum of Natural History and Science. https://books.google.com/books?id=S--oDQAAQBAJ.
- ↑ Soibelzon, Leopoldo H.; Pomi, Lucas H.; Tonni, Eduardo P.; Rodriguez, Sergio; Dondas, Alejandro (2009-09-01). "First report of a South American short-faced bears' den (Arctotherium angustidens): palaeobiological and palaeoecological implications". Alcheringa: An Australasian Journal of Palaeontology 33 (3): 211–222. doi:10.1080/03115510902844418. ISSN 0311-5518. Bibcode: 2009Alch...33..211S. http://sedici.unlp.edu.ar/handle/10915/5364.
- ↑ 99.0 99.1 Czaplewski, Nicholas; Rogers, Kyler; Russell, Clayton (2018-06-01). "Late pleistocene vertebrates from three-forks cave, Adair county, Oklahoma Ozark highland". Journal of Cave and Karst Studies 80 (2): 1–16. doi:10.4311/2017PA0118. https://www.researchgate.net/publication/324068143.
- ↑ 100.0 100.1 Puckette, William L. (1976). "Notes on the occurrence of the short-faced bear (Arctodus) in Oklahoma". Proceedings of the Oklahoma Academy of Science 56: 67–68.
- ↑ 101.0 101.1 101.2 101.3 101.4 Kurten, B.; Anderson, Elaine (1974). "Association of Ursus arctos and Arctodus simus (Mammalia: Ursidae) in the late Pleistocene of Wyoming". Breviora 426: 1––6. ISSN 0006-9698. https://www.biodiversitylibrary.org/part/32631.
- ↑ 102.0 102.1 Jones, D. Brent; Desantis, Larisa R. G. (2016-06-29). "Dietary Ecology of the Extinct Cave Bear: Evidence of Omnivory as Inferred from Dental Microwear Textures". Acta Palaeontologica Polonica 61 (4): 735–741. doi:10.4202/app.00253.2016. ISSN 0567-7920.
- ↑ 103.0 103.1 103.2 103.3 DeSantis, Larisa; Schubert, Blaine; Schmitt-Linville, Elizabeth; Ungar, Peter; Donohue, Shelly; Haupt, Ryan (2015-01-01). "Dental Microwear Textures of Carnivorans from the La Brea Tar Pits, California, and Potential Extinction Implications". La Brea and Beyond: The Paleontology of Asphalt-Preserved Biotas: 37–52. https://www.researchgate.net/publication/282253545.
- ↑ Carbone, Chris; Teacher, Amber; Rowcliffe, J. Marcus (2007-01-16). "The Costs of Carnivory" (in en). PLOS Biology 5 (2). doi:10.1371/journal.pbio.0050022. ISSN 1545-7885. PMID 17227145.
- ↑ Smith, Felisa A.; Boyer, Alison G.; Brown, James H.; Costa, Daniel P.; Dayan, Tamar; Ernest, S. K. Morgan; Evans, Alistair R.; Fortelius, Mikael et al. (2010-11-26). "The Evolution of Maximum Body Size of Terrestrial Mammals" (in en). Science 330 (6008): 1216–1219. doi:10.1126/science.1194830. ISSN 0036-8075. PMID 21109666. Bibcode: 2010Sci...330.1216S. https://www.science.org/doi/10.1126/science.1194830.
- ↑ Lauriol, Bernard; Gotthardt, Ruth (2016). Leduc, Heather. ed. The Ni'iinlii Njik Caves, Northern Yukon. Government of Yukon. ISBN 978-1-55362-752-4. https://emrlibrary.gov.yk.ca/Tourism/ni'iinlii-njik-caves-northern-yukon-2016.pdf.
- ↑ Soibelzon, Leopoldo H.; Grinspan, Gustavo A.; Bocherens, Hervé; Acosta, Walter G.; Jones, Washington; Blanco, Ernesto R.; Prevosti, Francisco (November 2014). "South American giant short-faced bear (Arctotherium angustidens) diet: evidence from pathology, morphology, stable isotopes, and biomechanics" (in en). Journal of Paleontology 88 (6): 1240–1250. doi:10.1666/13-143. ISSN 0022-3360. Bibcode: 2014JPal...88.1240S. https://www.cambridge.org/core/journals/journal-of-paleontology/article/abs/south-american-giant-shortfaced-bear-arctotherium-angustidens-diet-evidence-from-pathology-morphology-stable-isotopes-and-biomechanics/2616CEF2B348B1453C68EF26CF2858C9.
- ↑ 108.00 108.01 108.02 108.03 108.04 108.05 108.06 108.07 108.08 108.09 108.10 108.11 108.12 108.13 Bocherens, Hervé (2015-06-01). "Isotopic tracking of large carnivore palaeoecology in the mammoth steppe" (in en). Quaternary Science Reviews 117: 42–71. doi:10.1016/j.quascirev.2015.03.018. ISSN 0277-3791. Bibcode: 2015QSRv..117...42B. https://www.sciencedirect.com/science/article/pii/S0277379115001250.
- ↑ 109.0 109.1 109.2 109.3 109.4 109.5 109.6 109.7 Mychajliw, Alexis M.; Rick, Torben C.; Dagtas, Nihan D.; Erlandson, Jon M.; Culleton, Brendan J.; Kennett, Douglas J.; Buckley, Michael; Hofman, Courtney A. (2020-09-16). "Biogeographic problem-solving reveals the Late Pleistocene translocation of a short-faced bear to the California Channel Islands". Scientific Reports 10 (1): 15172. doi:10.1038/s41598-020-71572-z. PMID 32938967.
- ↑ 110.0 110.1 110.2 Smith, Felisa A.; Elliott Smith, Emma A.; Villaseñor, Amelia; Tomé, Catalina P.; Lyons, S. Kathleen; Newsome, Seth D. (2022-09-27). "Late Pleistocene megafauna extinction leads to missing pieces of ecological space in a North American mammal community" (in en). Proceedings of the National Academy of Sciences 119 (39). doi:10.1073/pnas.2115015119. ISSN 0027-8424. PMID 36122233. Bibcode: 2022PNAS..11915015S.
- ↑ 111.00 111.01 111.02 111.03 111.04 111.05 111.06 111.07 111.08 111.09 Kubiak, Cara; Grimes, Vaughan; Van Biesen, Geert; Keddie, Grant; Buckley, Mike; Macdonald, Reba; Richards, M. P. (2022-06-27). "Dietary niche separation of three Late Pleistocene bear species from Vancouver Island, on the Pacific Northwest Coast of North America" (in en). Journal of Quaternary Science 38: 8–20. doi:10.1002/jqs.3451. ISSN 0267-8179. https://onlinelibrary.wiley.com/doi/10.1002/jqs.3451.
- ↑ 112.0 112.1 112.2 112.3 112.4 Fox-Dobbs, Kena; Leonard, Jennifer A.; Koch, Paul L. (2008-04-24). "Pleistocene megafauna from eastern Beringia: Paleoecological and paleoenvironmental interpretations of stable carbon and nitrogen isotope and radiocarbon records" (in en). Palaeogeography, Palaeoclimatology, Palaeoecology 261 (1): 30–46. doi:10.1016/j.palaeo.2007.12.011. ISSN 0031-0182. Bibcode: 2008PPP...261...30F. https://www.sciencedirect.com/science/article/pii/S0031018208000266.
- ↑ Daeschler, Edward B. (1996-12-31), "Selective mortality of mastodons (Mammut americanum) from the Port Kennedy Cave (Pleistocene; lrvingtonian), Montgomery County, Pennsylvania", Palaeoecology and Palaeoenvironments of Late Cenozoic Mammals (University of Toronto Press): pp. 83–96, doi:10.3138/9781487574154-008, ISBN 978-1-4875-7415-4, https://www.degruyter.com/document/doi/10.3138/9781487574154-008/html, retrieved 2024-01-23
- ↑ 114.0 114.1 "A Baby Mastodon Deathtrap (?)" (in en). 2010-02-17. https://www.nationalgeographic.com/science/article/a-baby-mastodon-deathtrap.
- ↑ Wilkins, William; Pagnac, Darrin; Ellingsen, Marius (31 October 2013). "Chewing the Fat with Arctodus: Collaborative Undergraduate Development of a Calibrate Apparatus for Use in Bite Force Research". Journal of Vertebrate Paleontology 73rd Meeting: 238. https://vertpaleo.org/wp-content/uploads/2021/03/SVP-2013-merged-book-10-15-2013.pdf.
- ↑ Guthrie, R. Dale (1988). Mead, Jim I.. ed. "Bone Litter from an Alaskan Pleistocene Carnivore Den". Current Research in the Pleistocene 5: 84–85. https://liberalarts.tamu.edu/csfa/wp-content/uploads/sites/14/2023/07/CRP-5-1988.pdf.
- ↑ Sattler, Robert A. (1997). "Large Mammals in Lower Rampart Cave 1, Alaska: Interspecific Utilization of an Eastern Beringian Cave". Geoarchaeology 12 (6): 657–688. doi:10.1002/(SICI)1520-6548(199709)12:6<657::AID-GEA7>3.0.CO;2-Y. Bibcode: 1997Gearc..12..657S.
- ↑ 118.00 118.01 118.02 118.03 118.04 118.05 118.06 118.07 118.08 118.09 118.10 Salis, Alexander T; Bray, Sarah C E; Lee, Michael S Y; Heiniger, Holly; Barnett, Ross; Burns, James A; Doronichev, Vladimir; Fedje, Daryl; Golovanova, Liubov; Harington, C Richard; Hockett, Bryan; Kosintsev, Pavel; Lai, Xulong; Mackie, Quentin; Vasiliev, Sergei; Weinstock, Jacobo; Yamaguchi, Nobuyuki; Meachen, Julie; Cooper, Alan; Mitchell, Kieren J (3 September 2020). "Lions and brown bears colonized North America in multiple synchronous waves of dispersal across the Bering Land Bridge". bioRxiv 10.1101/2020.09.03.279117.
- ↑ 119.0 119.1 119.2 Redmond, Brian G.; Tankersley, Kenneth B. (10 February 2005). "Evidence of Early Paleoindian Bone Modification and Use at the Sheriden Cave Site (33WY252), Wyandot County, Ohio" (in en). American Antiquity 70 (3): 503–526. doi:10.2307/40035311. ISSN 0002-7316. https://www.cambridge.org/core/product/identifier/S0002731600039020/type/journal_article.
- ↑ 120.0 120.1 Bocherens, H.; Emslie, S. D.; Billiou, D.; Mariotti, A. (1995). "Stables isotopes (13C, 15N) and paleodiet of the giant short-faced bear (Arctodus simus)". Comptes rendus de l'Académie des Sciences 320 (8): 779–784. ISSN 1251-8050. https://www.academia.edu/751564.
- ↑ Rawlence, Nicolas J.; Wood, Jamie R.; Bocherens, Herve; Rogers, Karyne M. (2016-06-15). "Dietary interpretations for extinct megafauna using coprolites, intestinal contents and stable isotopes: Complimentary or contradictory?". Quaternary Science Reviews 142: 173–178. doi:10.1016/j.quascirev.2016.05.017. ISSN 0277-3791. Bibcode: 2016QSRv..142..173R. https://www.sciencedirect.com/science/article/pii/S0277379116301548.
- ↑ 122.0 122.1 122.2 122.3 Kohn, Matthew J.; McKay, Moriah P. (2012-04-01). "Paleoecology of late Pleistocene–Holocene faunas of eastern and central Wyoming, USA, with implications for LGM climate models". Palaeogeography, Palaeoclimatology, Palaeoecology 326-328: 42–53. doi:10.1016/j.palaeo.2012.01.037. ISSN 0031-0182. Bibcode: 2012PPP...326...42K. https://www.sciencedirect.com/science/article/pii/S0031018212000612.
- ↑ Fisher, Daniel C.; Cherney, Michael D.; Newton, Cody; Rountrey, Adam N.; Calamari, Zachary T.; Stucky, Richard K.; Lucking, Carol; Petrie, Lesley (November 2014). "Taxonomic overview and tusk growth analyses of Ziegler Reservoir proboscideans" (in en). Quaternary Research 82 (3): 518–532. doi:10.1016/j.yqres.2014.07.010. ISSN 0033-5894. Bibcode: 2014QuRes..82..518F. https://www.cambridge.org/core/journals/quaternary-research/article/abs/taxonomic-overview-and-tusk-growth-analyses-of-ziegler-reservoir-proboscideans/AF05EC53DB12810410A63CBD6A9FB6BD.
- ↑ 124.0 124.1 Trayler, Robin Brendan (December 2012). "Stable Isotope Records of Inland California Megafauna- New Insights Into Pleistocene Paleoecology and Paleoenvironmental Conditions (Masters Thesis)". College of Science and Mathematics, California State University Fresno. https://scholarworks.calstate.edu/downloads/h128nf901.
- ↑ 125.0 125.1 Chatters, James C.; Potter, Ben A.; Fiedel, Stuart J.; Morrow, Juliet E.; Jass, Christopher N.; Wooller, Matthew J. (2024-12-04). "Mammoth featured heavily in Western Clovis diet". Science Advances 10 (49). doi:10.1126/sciadv.adr3814. PMID 39630905. Bibcode: 2024SciA...10R3814C.
- ↑ Lanoë, François B.; Reuther, Joshua D.; Holmes, Charles E.; Hodgins, Gregory W. L. (2017-11-01). "Human paleoecological integration in subarctic eastern Beringia" (in en). Quaternary Science Reviews 175: 85–96. doi:10.1016/j.quascirev.2017.10.003. ISSN 0277-3791. Bibcode: 2017QSRv..175...85L.
- ↑ Grumet, Robert S. (September 2000). Bay, Plain, and Piedmont- A Landscape History of the Chesapeake Heartland from 1.3 Billion Years Ago to 2000. The Chesapeake Bay Heritage Context Project. pp. 16, 21, 167.
- ↑ Morgan, Gary S.; Lucas, Spencer G. (2005). "Pleistocene vertebrate faunas in New Mexico from alluvial, fluvial, and lacustrine deposits". New Mexico Museum of Natural History and Science Bulletin 28: 207. https://www.researchgate.net/publication/281207462.
- ↑ 129.0 129.1 129.2 Albright, L. Barry; Sanders, Albert; Weems, Robert; Cicimurri, David; Knight, James (2020-01-24). "Cenozoic Vertebrate Biostratigraphy of South Carolina, USA, and Additions to the Fauna". Showcase of Faculty Scholarly & Creative Activity. https://digitalcommons.unf.edu/facultyshowcase/2020/Showcase/1.
- ↑ Dalquest, W. W.; Mooser, O. (1980-12-19). "Arctodus pristinus Leidy in the Pleistocene of Aguascalientes, Mexico". Journal of Mammalogy 61 (4): 724–725. doi:10.2307/1380320.
- ↑ Berta, Annalisa (1995). "Fossil carnivores from the Leisley Shell Pit, Hillsborough County, Florida". Bulletin of the Florida Museum of Natural History 37 Part II (14): 436–499. https://www.floridamuseum.ufl.edu/wp-content/uploads/sites/35/2017/03/Vol-37-No-14.pdf.
- ↑ Gould, G.C.; Quitmyer, Irvy (2005-01-01). "Titanis walleri: Bones of contention". Bulletin of the Florida Museum of Natural History 45 (4): 201–229. doi:10.58782/flmnh.xumx1681. https://www.researchgate.net/publication/288892560.
- ↑ MacFadden, Bruce; Labs-Hochstein, Joann; Hulbert, Richard; Baskin, Jon (2007-02-01). "Revised age of the late Neogene terror bird (Titanis) in North America during the Great American Interchange". Geology 35 (2): 123. doi:10.1130/G23186A.1. Bibcode: 2007Geo....35..123M. https://www.researchgate.net/publication/249521166.
- ↑ 134.0 134.1 Domínguez-Rodrigo, Manuel; Egeland, Charles P.; Cobo-Sánchez, Lucía; Baquedano, Enrique; Hulbert, Richard C. (2022-05-02). "Sabertooth carcass consumption behavior and the dynamics of Pleistocene large carnivoran guilds" (in en). Scientific Reports 12 (1): 6045. doi:10.1038/s41598-022-09480-7. ISSN 2045-2322. PMID 35501323. Bibcode: 2022NatSR..12.6045D.
- ↑ Legge, A. J. (March 1991). "Where ends meat" (in en). Antiquity 65 (246): 147–153. doi:10.1017/S0003598X00079424. ISSN 0003-598X. https://www.cambridge.org/core/journals/antiquity/article/abs/where-ends-meat/21B26626DA3AC3377EDF5CEB58A6D64A.
- ↑ 136.0 136.1 "The Giant Short-Faced Bear". 2018-03-02. https://bear.org/the-giant-short-faced-bear/.
- ↑ "great short-faced bear fossil". https://www.backyardnature.net/loess/~s-fbear.htm.
- ↑ 138.0 138.1 138.2 138.3 138.4 138.5 138.6 Paul, Matheus (2001). "Pleistocene carnivores and humans in eastern Beringia: did short-faced bears really keep people out of North America?". in Gerlach, S. Craig (in en). People and Wildlife in Northern North America: Essays in honor of R. Dale Guthrie. BAR Publishing. pp. 79–101. doi:10.30861/9781841712369. ISBN 978-1-4073-5292-3. https://www.fulcrum.org/concern/monographs/9z9031660.
- ↑ 139.0 139.1 Holliday, Vance; Surovell, Todd; Meltzer, David; Grayson, Donald; Boslough, Mark (2014-08-01). "The Younger Dryas impact hypothesis: A cosmic catastrophe". Journal of Quaternary Science 29 (6): 515–530. doi:10.1002/jqs.2724. Bibcode: 2014JQS....29..515H. https://www.researchgate.net/publication/265132201.
- ↑ 140.0 140.1 140.2 Faith, J. Tyler; Surovell, Todd A. (2009-12-08). "Synchronous extinction of North America's Pleistocene mammals". Proceedings of the National Academy of Sciences 106 (49): 20641–20645. doi:10.1073/pnas.0908153106. ISSN 0027-8424. PMID 19934040. Bibcode: 2009PNAS..10620641F.
- ↑ Pichardo, Mario (2003). "Overview of Central Mexican Prehistory: Morphostratigraphy, Chronostratigraphy, Biostratigraphy". Anthropologischer Anzeiger 61 (2): 141–174. doi:10.1127/anthranz/61/2003/141. ISSN 0003-5548. PMID 12872543.
- ↑ "KGS--Guidebook 5--Wisconsinan Mammalian Faunas". https://www.kgs.ku.edu/Publications/Bulletins/GB5/Martin2/.
- ↑ Firby, Jean Brower (1968) (in en). Revision of the Middle Pleistocene Irvington Fauna of California. University of California. https://books.google.com/books?id=qOdKAQAAMAAJ.
- ↑ III, Alois F. Pajak; Scott, Eric; Bell, Christopher J. (13 September 1996). "A review of the biostratigraphy of Pliocene and Pleistocene sediments in the Elsinore Fault Zone, Riverside County, California". PaleoBios 17 (2–4): 28–49. https://www.academia.edu/download/69346012/A_review_of_the_biostratigraphy_of_Plioc20210910-25423-3lhn5q.pdf.
- ↑ "Elsinore Fault Zone". https://www.utep.edu/leb/pleistnm/sites/elsinore.htm.
- ↑ Dundas, Robert G.; Chatters, James C. (2013-01-01). "The mid-Irvingtonian Fairmead Landfill fossil site, Madera County Paleontology Collection, and Fossil Discovery Center of Madera County, California". in Keith Putirka (in en). Geologic Excursions from Fresno, California, and the Central Valley: A Tour of California's Iconic Geology. Geological Society of America. pp. 63–78. doi:10.1130/2013.0032(04). ISBN 978-0-8137-0032-8.
- ↑ "San Timoteo Badlands". https://www.utep.edu/leb/pleistnm/sites/santimoteo.htm.
- ↑ 148.0 148.1 148.2 148.3 Schubert, Blaine; Cox, Shelley; Coltrain, Joan (31 October 2013). "The Significance of Rancho La Brea for Interpreting the Paleobiology of Giant Short-Faced Bears". Society of Vertebrate Paleontology 73rd Meeting: 207. https://vertpaleo.org/wp-content/uploads/2021/03/SVP-2013-merged-book-10-15-2013.pdf.
- ↑ Tar Pits Fossil Count. La Brea Tar Pits and Museum. https://tarpits.org/sites/default/files/2019-04/tar_pits_fossil_count.pdf.
- ↑ Carbone, Chris; Maddox, Tom; Funston, Paul J.; Mills, Michael G.L.; Grether, Gregory F.; Van Valkenburgh, Blaire (2009-02-23). "Parallels between playbacks and Pleistocene tar seeps suggest sociality in an extinct sabretooth cat, Smilodon". Biology Letters 5 (1): 81–85. doi:10.1098/rsbl.2008.0526. ISSN 1744-9561. PMID 18957359.
- ↑ Springer, Kathleen; Scott, Eric; Murray, Lyndon K.; Sagebiel, James (2009). Albright, L. B. III. ed. "The Diamond Valley Lake local fauna: late Pleistocene vertebrates from inland southern California". Papers on Geology, Vertebrate Paleontology, and Biostratigraphy in Honor of Michael O. Woodburne. https://www.academia.edu/218818.
- ↑ 152.0 152.1 152.2 152.3 152.4 University of Geneva, Switzerland; Ray, N.; Adams, J.M. (2001). "A GIS-based Vegetation Map of the World at the Last Glacial Maximum (25,000-15,000 BP)". Internet Archaeology (11). doi:10.11141/ia.11.2. http://intarch.ac.uk/journal/issue11/rayadams_index.html.
- ↑ 153.0 153.1 Goebel, Ted; Hockett, Bryan; Adams, Kenneth D.; Rhode, David; Graf, Kelly (2011-10-15). "Climate, environment, and humans in North America's Great Basin during the Younger Dryas, 12,900–11,600 calendar years ago". Quaternary International. Humans and Younger Dryas: Dead End, Short Detour, Or Open Road to the Holocene? 242 (2): 479–501. doi:10.1016/j.quaint.2011.03.043. ISSN 1040-6182. Bibcode: 2011QuInt.242..479G. https://www.sciencedirect.com/science/article/pii/S1040618211001893.
- ↑ Long, C. A. (1971). "Significance of the Late Pleistocene fauna from the Little Box Elder Cave, Wyoming, to studies of zoogeography of recent mammals" (in en). Great Basin Naturalist 31 (2).
- ↑ Walker, Danny N. (1987). "Late Pleistocene/Holocene Environmental Changes in Wyoming: the Mammalian Record". Office of the Wyoming State Archeologist. https://www.uwyo.edu/anthropology/_files/docs/walker/26%20walker%201987%20environmental%20summary.pdf.
- ↑ Smith, Larry N.; Hill, Christopher L.; Reiten, Jon. "Quaternary and Late Tertiary of Montana: Climate, Glaciation, Stratigraphy, and Vertebrate Fossils". Montana Bureau of Mines and Geology Publication 122 1: Geologic History. https://mbmg.mtech.edu/pdf/geologyvolume/Smith_QuaternaryMontanaFinal.pdf.
- ↑ Hill, Christopher L. (2006-01-01). "Stratigraphic and geochronologic contexts of mammoth (Mammuthus) and other Pleistocene fauna, Upper Missouri Basin (northern Great Plains and Rocky Mountains), U.S.A." (in en). Quaternary International. Third International Mammoth Conference, Dawson, Yukon 142-143: 87–106. doi:10.1016/j.quaint.2005.03.007. ISSN 1040-6182. Bibcode: 2006QuInt.142...87H. https://www.sciencedirect.com/science/article/pii/S104061820500056X.
- ↑ 158.0 158.1 Nelson, Michael E.; Madsen, James H. (1983). "A Giant Short-Faced Bear (Arctodus simus) from the Pleistocene of Northern Utah". Transactions of the Kansas Academy of Science 86 (1): 1–9. doi:10.2307/3628418.
- ↑ Cassiliano, Michael L. (1999). "Biostratigraphy of Blancan and Irvingtonian Mammals in the Fish Creek-Vallecito Creek Section, Southern California, and a Review of the Blancan-Irvingtonian Boundary". Journal of Vertebrate Paleontology 19 (1): 169–186. doi:10.1080/02724634.1999.10011131. ISSN 0272-4634.
- ↑ Murray, Lyndon Keith (December 2008). "Effects of taxonomic and locality inaccuracies on biostratigraphy and biochronology of the Hueso and Tapiado formations in the Vallecito Creek-Fish Creek section, Anza-Borrego Desert, California". University of Texas at Austin. http://hdl.handle.net/2152/15340.
- ↑ "Abstract of Papers. Fifty-Seventh Annual Meeting, Society of Vertebrate Paleontology". Journal of Vertebrate Paleontology 17 (3): A1–A93. 1997. ISSN 0272-4634. https://www.jstor.org/stable/4523848.
- ↑ Grayson, Donald K. (2006-11-01). "The Late Quaternary biogeographic histories of some Great Basin mammals (western USA)" (in en). Quaternary Science Reviews 25 (21): 2964–2991. doi:10.1016/j.quascirev.2006.03.004. ISSN 0277-3791. Bibcode: 2006QSRv...25.2964G. https://www.sciencedirect.com/science/article/pii/S0277379106001405.
- ↑ "U-Bar Cave". https://www.utep.edu/leb/pleistnm/sites/ubarcave.htm.
- ↑ Schultz, C. Bertrand; Howard, Edgar B.; Schultz, C. Bernard (1935). "The Fauna of Burnet Cave, Guadalupe Mountains, New Mexico". Proceedings of the Academy of Natural Sciences of Philadelphia 87: 273–298. ISSN 0097-3157.
- ↑ Harris, Arthur H. (1993). "Quaternary Vertebrates of New Mexico". Vertebrate Paleontology in New Mexico, New Mexico Museum of Natural History and Science Bulletin 2: 179–197. https://www.utep.edu/leb/curators/QuatVert.pdf.
- ↑ Harris, A. H.; Findley, J. S. (1964-01-01). "Pleistocene-Recent fauna of the Isleta caves, Bernalillo County, New Mexico" (in en). American Journal of Science 262 (1): 114–120. doi:10.2475/ajs.262.1.114. ISSN 0002-9599. Bibcode: 1964AmJS..262..114H.
- ↑ Morgan, Gary S.; Lucas, Spencer G.; Love, David (2009). "Cenozoic vertebrates from Socorro County, central New Mexico". in Virgil Lueth. pp. 321–336. https://nmgs.nmt.edu/publications/guidebooks/downloads/60/60_p0321_p0336.pdf.
- ↑ Morgan, Gary S.; Lucs, Spencer G. (2005-01-01). "Pleistocene vertebrates from southeastern New Mexico". KIP Articles 2006. https://digitalcommons.usf.edu/kip_articles/4347.
- ↑ Jefferson, George T. (2003), "Stratigraphy and paleontology of the middle to late Pleistocene Manix Formation, and paleoenvironments of the central Mojave River, Southern California" (in en), Paleoenvironments and paleohydrology of the Mojave and southern Great Basin deserts (Geological Society of America), doi:10.1130/0-8137-2368-x.43, ISBN 978-0-8137-2368-6, https://pubs.geoscienceworld.org/books/book/509/chapter/3800872, retrieved 2024-07-18
- ↑ "Catalogue of Late Quaternary Vertebrates from California". City of Santee. 26 March 2011. http://sntbberry.cityofsanteeca.gov/sites/FanitaRanch/Public/Remainder%20of%20the%20Record/(2)%20Reference%20Documents%20from%20EIR%20&%20Technical%20Reports/Tab%20081%20-%201991%20Jefferson%20-%20Catalogue%20of%20Late%20Quaternary%20Vertebrates%20(revised%202020).pdf.
- ↑ Emslie, Steven D.; Mead, Jim I. (August 2020). "The Age and Vertebrate Paleontology of Labor-of-Love Cave, White Pine County, Nevada". Western North American Naturalist 80 (3): 277–291. doi:10.3398/064.080.0301. ISSN 1527-0904. Bibcode: 2020WNAN...80..301E. https://bioone.org/journals/western-north-american-naturalist/volume-80/issue-3/064.080.0301/The-Age-and-Vertebrate-Paleontology-of-Labor-of-Love-Cave/10.3398/064.080.0301.full.
- ↑ "Late Pleistocene Airport Lane Fossil Site, La Grande..." (in en). https://www.yumpu.com/en/document/view/12116022/late-pleistocene-airport-lane-fossil-site-la-grande-.
- ↑ Van Tassell, Jay; Rinehart, John; Mahrt, Laura (June 2014). "Late Pleistocene Airport Lane fossil site, La Grande, northeast Oregon". Oregon Geology 70 (1): 3–13. https://www.oregongeology.org/pubs/OG/OGv70n01_print.pdf.
- ↑ "The Spokesman-Review - Google News Archive Search". https://news.google.com/newspapers?nid=1314&dat=19280626&id=PGpWAAAAIBAJ&sjid=vvMDAAAAIBAJ&pg=5453,5034598.
- ↑ Harris, Arthur (2014-08-30). Pleistocene Vertebrates of Southwestern USA and Northwestern Mexico. https://www.researchgate.net/publication/265165536.
- ↑ Harris, Arthur H.. "Reconstruction of Mid Wisconsin Environments in Southern New Mexico". National Geographic Research. https://www.wipp.energy.gov/library/cra/2009_cra/references/Others/Harris_1987_Reconstruction_of_Mid_Wisconsin_Environments.pdf.
- ↑ 177.0 177.1 Eng-Ponce, Joaquin (August 2021). "Reconstruccion paeloambiental del yacimiento La Cinta-Portalitos, Michoacan-Guanajuato, Mexico (thesis)". Faculty of Biology, Universidad Michoacana de San Nicolás de Hidalgo. http://bibliotecavirtual.dgb.umich.mx:8083/xmlui/bitstream/handle/DGB_UMICH/6432/FB-M-2021-0909.pdf.
- ↑ Lucas, Spencer G. (January 2008). "Late Pleistocene Vertebrate Fossil Assemblages From Jalisco, Mexico". Neogene Mammals. New Mexico Museum of Natural History and Science Bulletin 44: 51–64. https://www.researchgate.net/publication/281862788.
- ↑ Hibbard, Claude W. (18 February 1955). "Pleistocene Vertebrates from the Upper Becerra (Becerra Superior) Formation, Valley of Tequixquiac, Mexico, with Notes on Other Pleistocene Forms" (in en-US). Contributions from the Museum of Paleontology XII (5): 47–96. http://deepblue.lib.umich.edu/handle/2027.42/48290.
- ↑ Carranza-Castañeda, Oscar; Miller, Wade E. (16 September 1987). "Rediscovered type specimens and other important published Pleistocene mammalian fossils from Central Mexico". Journal of Vertebrate Paleontology 7 (3): 335–341. doi:10.1080/02724634.1987.10011664. Bibcode: 1987JVPal...7..335C.
- ↑ Gobetz, Katrina E.; Martin, Larry D. (2001). "An Exceptionally Large Short-faced Bear (Arctodus simus) from the Late Pleistocene(?) /Early Holocene of Kansas". Current Research in the Pleistocene 18: 97–99. ISSN 8755-898X. https://liberalarts.tamu.edu/csfa/wp-content/uploads/sites/14/2023/07/CRP-18-2001.pdf.
- ↑ "Abstract: Pleistocene Vertebrates From the Doeden Local Fauna (Illinoian/Sangamonian?), Yellowstone River Valley, Eastern Montana (Rocky Mountain - 55th Annual Meeting (May 7-9, 2003))". https://gsa.confex.com/gsa/2003RM/webprogram/Paper53111.html.
- ↑ McDonald, Andrew T.; Atwater, Amy L.; Dooley Jr, Alton C.; Hohman, Charlotte J.H. (2020-11-16). "The easternmost occurrence of Mammut pacificus (Proboscidea: Mammutidae), based on a partial skull from eastern Montana, USA". PeerJ 8. doi:10.7717/peerj.10030. ISSN 2167-8359. PMID 33240588.
- ↑ Hill, Christopher L; Wilson, Michael C (2002). "Fossil Arctodus from the Doeden Local Fauna (Illinoian/Sangamonian?), Eastern Montana". Unknown. https://www.researchgate.net/publication/270216214.
- ↑ Froese, Duane; Stiller, Mathias; Heintzman, Peter D.; Reyes, Alberto V.; Zazula, Grant D.; Soares, André E. R.; Meyer, Matthias; Hall, Elizabeth et al. (2017-03-28). "Fossil and genomic evidence constrains the timing of bison arrival in North America" (in en). Proceedings of the National Academy of Sciences 114 (13): 3457–3462. doi:10.1073/pnas.1620754114. ISSN 0027-8424. PMID 28289222. Bibcode: 2017PNAS..114.3457F.
- ↑ 186.0 186.1 Louguet-Lefebvre, Sophie (2013-12-15). "The Columbian mammoths from the Upper Pleistocene of Hot Springs (South Dakota, United States)" (in en). PALEO. Revue d'archéologie préhistorique 24 (24): 149–171. doi:10.4000/paleo.2861. ISSN 1145-3370.
- ↑ Johnson, Eileen (1986). "Late Pleistocene and Early Holocene Vertebrates and Paleoenvironments on the Southern High Plains, U.S.A.". Géographie physique et Quaternaire 40 (3): 249–261. doi:10.7202/032647ar. https://www.erudit.org/en/journals/gpq/1900-v1-n1-gpq1924/032647ar.pdf.
- ↑ Smith, Felisa A.; Tomé, Catalina P.; Elliott Smith, Emma A.; Lyons, S. Kathleen; Newsome, Seth D.; Stafford, Thomas W. (February 2016). "Unraveling the consequences of the terminal Pleistocene megafauna extinction on mammal community assembly" (in en). Ecography 39 (2): 223–239. doi:10.1111/ecog.01779. ISSN 0906-7590. Bibcode: 2016Ecogr..39..223S. https://onlinelibrary.wiley.com/doi/10.1111/ecog.01779.
- ↑ "Giant Short-Faced Bear | University of Iowa Museum of Natural History - The University of Iowa" (in en). https://mnh.uiowa.edu/giant-short-faced-bear.
- ↑ Taylor, D. W. (1960). "Late Cenozoic molluscan faunas from the High Plains". USGS Report: 4. doi:10.3133/pp337. ISSN 2330-7102. Bibcode: 1960usgs.rept....4T. https://pubs.er.usgs.gov/publication/pp337.
- ↑ Harington, C. R. (1973). "A Short-Faced Bear From Ice Age Deposits at Lebret, Saskatchewan" (in en). Blue Jay 31 (1). doi:10.29173/bluejay4039. ISSN 2562-5667. https://bluejayjournal.ca/index.php/bluejay/article/view/4039.
- ↑ Harington, C. (1990). "Vertebrates of the Last Interglaciation in Canada: A Review, with New Data" (in en). Géographie physique et Quaternaire 44 (3): 375–387. doi:10.7202/032837ar. ISSN 0705-7199.
- ↑ Burns, James A.; Young, Robert R. (1994-02-01). "Pleistocene mammals of the Edmonton area, Alberta. Part I. The carnivores" (in en). Canadian Journal of Earth Sciences 31 (2): 393–400. doi:10.1139/e94-036. ISSN 0008-4077. Bibcode: 1994CaJES..31..393B. http://www.nrcresearchpress.com/doi/10.1139/e94-036.
- ↑ Young, Robert R.; Burns, James A.; Smith, Derald G.; Arnold, L. David; Rains, R. Bruce (1994-08-01). "A single, late Wisconsin, Laurentide glaciation, Edmonton area and southwestern Alberta 2.3.CO;2". Geology 22 (8): 683–686. doi:10.1130/0091-7613(1994)022<0683:ASLWLG>2.3.CO;2. ISSN 0091-7613.
- ↑ 195.0 195.1 Tankersley, Kenneth B. (26 May 1997). "Sheriden: A Clovis cave site in eastern North America". Geoarchaeology 12 (6): 713–724. doi:10.1002/(SICI)1520-6548(199709)12:6<713::AID-GEA9>3.0.CO;2-1. Bibcode: 1997Gearc..12..713T. https://onlinelibrary.wiley.com/doi/abs/10.1002/(SICI)1520-6548(199709)12:6%3C713::AID-GEA9%3E3.0.CO;2-1.
- ↑ Wilson, Ronald C. (1985). "Vertebrate Remains in Kentucky Caves". in Percy H. Dougherty. Caves and Karst of Kentucky. Series XI. 12. Kentucky Geological Survey, University of Kentucky Lexington. p. 171. https://kgs.uky.edu/kgsweb/olops/pub/kgs/KGS11SP12reduce.pdf.
- ↑ Colburn, Mona L. (July 2005). "Paleontological Inventory Project: Vertebrate Remains Found in Select Passages and Caves at Mammoth Cave National Park, Kentucky". Illinois State Museum: 271, 281, 292. http://npshistory.com/publications/maca/vertebrate-remains.pdf.
- ↑ Hawksley, Oscar (July 1965). "Short-Faced Bear (Arctodus) Fossils from Ozark Caves". Bulletin of the National Speleological Society 27 (3): 77–92. https://caves.org/pub/journal/NSS%20Bulletin/vol%2027%20part%203.pdf.
- ↑ Woodruff, Aaron L. (2016). "Description, Taphonomy, and Paleoecology of the Late Pleistocene Peccaries (Artiodactyla: Tayassuidae) from Bat Cave, Pulaski County, Missouri". Department of Geosciences, East Tennessee State University (Paper 3051). https://dc.etsu.edu/cgi/viewcontent.cgi?article=4444&context=etd.
- ↑ Woodruff, Aaron L.; Schubert, Blaine W. (2019-07-04). "Seasonal denning behavior and population dynamics of the late Pleistocene peccary Platygonus compressus (Artiodactyla: Tayassuidae) from Bat Cave, Missouri". PeerJ 7. doi:10.7717/peerj.7161. ISSN 2167-8359. PMID 31308997.
- ↑ Hawksley, Oscar; Reynolds, Jack F.; Foley, Robert F. (July 1973). "Pleistocene Vertebrate Fauna of Bat Cave, Pulaski County, Missouri". Bulletin of the National Speleological Society 35 (3): 61–87. https://caves.org/pub/journal/NSS%20Bulletin/Vol%2035%20num%203.pdf.
- ↑ Santucci, Vincent L.; Kenworthy, Jason; Kerbo, Ron (2022-01-18). "An inventory of paleontological resources associated with national park service caves". KIP Articles. https://digitalcommons.usf.edu/kip_articles/271.
- ↑ Smith, Matthew D; Dorale, Jeffrey A; Johnson, Aaron W; Forir, Matthew D (2013). "A speleothem record of paleoenvironmental change from Riverbluff Cave, Missouri, U.S.A". https://iro.uiowa.edu/esploro/outputs/abstract/A-speleothem-record-of-paleoenvironmental-change/9984240795902771.
- ↑ Simpson, Emily (2019-05-01). Paleoecology and Land-Use of Quaternary Megafauna from Saltville, Virginia (Master thesis). East Tennessee State University.
- ↑ Tucci, Harry J. (1986). "Archaeological Investigations of Durham Cave #2". Current Research in the Pleistocene 3: 25–26. https://liberalarts.tamu.edu/csfa/wp-content/uploads/sites/14/2023/07/CRP-3-1986.pdf.
- ↑ Ebersole, Jun A.; Ebersole, Sandy M. (December 2011). "Late Pleistocene Mammals of Alabama: A Comprehensive Faunal Review with 21 Previously Unreported Taxa". Alabama Museum of Natural History Bulletin 28: 24–25. http://almnh.museums.ua.edu/wp-content/uploads/sites/2/2018/12/BALMNH_No_28_2011.pdf.
- ↑ Baghai-Riding, Nina L.; Husley, Danielle B.; Beck, Christine; Blackwell, Eric (December 2017). "Late Pleistocene Megafauna from Mississippi Alluvium Plain Gravel Bars". Paludicola 11 (3): 124–147. https://paludicolavertpaleo.files.wordpress.com/2019/11/11-3-baghai-riding-2017.pdf.
- ↑ Ruddell, Michael W. (December 1999). "Quaternary Vertebrate Paleoecology of the Central Mississippi Alluvial Valley; Implications for the Initial Human Occupation". Tennessee Research and Creative Exchange. https://trace.tennessee.edu/cgi/viewcontent.cgi?article=3184&context=utk_graddiss.
- ↑ Kurtén, Björn; Kaye, John M. (March 1982). "Late Quaternary Carnivora from the Black Belt, Mississippi" (in en). Boreas 11 (1): 47–52. doi:10.1111/j.1502-3885.1982.tb00519.x. Bibcode: 1982Borea..11...47K. https://onlinelibrary.wiley.com/doi/10.1111/j.1502-3885.1982.tb00519.x.
- ↑ Kaye, John Morgan (1974). "Pleistocene Sediment and V ocene Sediment and Vertebrate Fossil Associations in the Mississippi Black Belt: a Genetic Approach". LSU Historical Dissertations and Theses. 2612. https://digitalcommons.lsu.edu/cgi/viewcontent.cgi?referer=&httpsredir=1&article=3611&context=gradschool_disstheses.
- ↑ Miller, Andrew (26 March 2021). "SC diver finds rare prehistoric bear tooth fossil in Cooper River" (in en). https://www.postandcourier.com/news/sc-diver-finds-rare-prehistoric-bear-tooth-fossil-in-cooper-river/article_c8cf9104-8d7a-11eb-bd4d-af15386d585a.html.
- ↑ Slaughter, Bob H. (1966). "The Moore Pit Local Fauna; Pleistocene of Texas". Journal of Paleontology 40 (1): 78–91. ISSN 0022-3360.
- ↑ David Webb, S.; Graham, Russell W.; Barnosky, Anthony D.; Bell, Christopher J.; Franz, Richard; Hadly, Elizabeth A.; Lundelius, Ernest L.; Gregory McDonald, H. et al. (2003) (in en), Vertebrate paleontology, Developments in Quaternary Sciences, 1, Elsevier, pp. 519–538, doi:10.1016/s1571-0866(03)01025-x, ISBN 978-0-444-51470-7, https://linkinghub.elsevier.com/retrieve/pii/S157108660301025X, retrieved 2022-06-28
- ↑ Harington, C. R. (1980). "Radiocarbon Dates on Some Quaternary Mammals and Artifacts from Northern North America". Arctic 33 (4): 815–832. doi:10.14430/arctic2598. ISSN 0004-0843.
- ↑ "The Ottawa naturalist: Vol. 25, no. 2 (May 1911) - Canadiana". https://www.canadiana.ca/view/oocihm.8_04906_273/9.
- ↑ 216.0 216.1 Mann, Daniel H.; Groves, Pamela; Kunz, Michael L.; Reanier, Richard E.; Gaglioti, Benjamin V. (2013-06-15). "Ice-age megafauna in Arctic Alaska: extinction, invasion, survival" (in en). Quaternary Science Reviews 70: 91–108. doi:10.1016/j.quascirev.2013.03.015. ISSN 0277-3791. Bibcode: 2013QSRv...70...91M. https://www.sciencedirect.com/science/article/pii/S0277379113001200.
- ↑ Monteath, Alistair J.; Gaglioti, Benjamin V.; Edwards, Mary E.; Froese, Duane (2021-12-28). "Late Pleistocene shrub expansion preceded megafauna turnover and extinctions in eastern Beringia" (in en). Proceedings of the National Academy of Sciences 118 (52). doi:10.1073/pnas.2107977118. ISSN 0027-8424. PMID 34930836. Bibcode: 2021PNAS..11807977M.
- ↑ 218.0 218.1 Murchie, Tyler J.; Monteath, Alistair J.; Mahony, Matthew E.; Long, George S.; Cocker, Scott; Sadoway, Tara; Karpinski, Emil; Zazula, Grant et al. (2021-12-08). "Collapse of the mammoth-steppe in central Yukon as revealed by ancient environmental DNA" (in en). Nature Communications 12 (1): 7120. doi:10.1038/s41467-021-27439-6. ISSN 2041-1723. PMID 34880234. Bibcode: 2021NatCo..12.7120M.
- ↑ 219.0 219.1 Barnes, I.; Matheus, P.; Shapiro, B.; Jensen, D.; Cooper, A. (2002-03-22). "Dynamics of Pleistocene Population Extinctions in Beringian Brown Bears" (in en). Science 295 (5563): 2267–2270. doi:10.1126/science.1067814. ISSN 0036-8075. PMID 11910112. Bibcode: 2002Sci...295.2267B. https://www.science.org/doi/10.1126/science.1067814.
- ↑ Leonard, Jennifer A.; Vilà, Carles; Fox-Dobbs, Kena; Koch, Paul L.; Wayne, Robert K.; Van Valkenburgh, Blaire (July 2007). "Megafaunal Extinctions and the Disappearance of a Specialized Wolf Ecomorph". Current Biology 17 (13): 1146–1150. doi:10.1016/j.cub.2007.05.072. ISSN 0960-9822. PMID 17583509. Bibcode: 2007CBio...17.1146L.
- ↑ Pedersen, Mikkel W.; Ruter, Anthony; Schweger, Charles; Friebe, Harvey; Staff, Richard A.; Kjeldsen, Kristian K.; Mendoza, Marie L. Z.; Beaudoin, Alwynne B. et al. (2016). "Postglacial viability and colonization in North America's ice-free corridor". Nature 537 (7618): 45–49. doi:10.1038/nature19085. PMID 27509852. Bibcode: 2016Natur.537...45P. http://eprints.gla.ac.uk/138297/1/138297.pdf.
- ↑ Weems, Robert E. (2018). "An Early Pleistocene (Early Irvingtonian) Footprint Fauna from the Bacons Castle Formation, Westmoreland Formation, Virginia". New Mexico Museum of Natural History and Science Bulletin 79: 731–748. https://www.researchgate.net/publication/348579743.
- ↑ 223.0 223.1 Lambert, W. David; Holling, Crawford S. (1998-03-01). "Original Articles: Causes of Ecosystem Transformation at the End of the Pleistocene: Evidence from Mammal Body-Mass Distributions". Ecosystems 1 (2): 157–175. doi:10.1007/s100219900012. ISSN 1432-9840. Bibcode: 1998Ecosy...1..157L. http://link.springer.com/10.1007/s100219900012.
- ↑ "The California grizzly bear | La Brea Tar Pits" (in en). https://tarpits.org/california-grizzly-bear.
- ↑ 225.0 225.1 Soibelzon, Leopoldo; Tarantini, Viviana Beatriz (January 2009). "Body mass estimation of extinct and extant South American bears (Ursidae, Tremarctinae)". Revista del Museo Argentino de Ciencias Naturales 11 (2): 243–254. doi:10.22179/REVMACN.11.263. https://www.researchgate.net/publication/279714500.
- ↑ 226.0 226.1 Arroyo-Cabrales, Joaquin; Johnson, Eileen; Graham, Russell W.; Pérez-Crespo, Victor (2016-07-24). North American ursid (mammalia: ursidae) defaunation from Pleistocene to recent. 33. ICBS Proceedings (Cranium). pp. 51–56. OCLC 1227719621. https://www.researchgate.net/publication/305681538.
- ↑ Holliday, Vance T.; Johnson, Eileen; Haas, Herbert; Stuckenrath, Robert (1983). "Radiocarbon Ages from the Lubbock Lake Site, 1950-1980: Framework for Cultural and Ecological Change on the Southern High Plains". Plains Anthropologist 28 (101): 165–182. doi:10.1080/2052546.1983.11909122. ISSN 0032-0447.
- ↑ Brian G. Redmond (March 2006). "Before the Western Reserve: An Archaeological History of Northeast Ohio". The Cleveland Museum of Natural History. p. 2. https://www.cmnh.org/CMNH/media/CMNH_Media/C-R%20Docs/BeforeWR.pdf.
- ↑ Kenworthy, Jason P.; Santucci, Vincent L.; McNerney, Michaleen; Snell, Kathryn (August 2005). "Paleontological Resource Inventory and Monitoring: Upper Columbian Basin Network". National Park Service (U.S. Department of the Interior) TIC# D-259: 51. http://npshistory.com/publications/nepe/paleontological-res-im-2005.pdf.
- ↑ Chadez, Jenifer; Sappington, Robert Lee (2017-12-01). "The Holocene exploitation of mammals in the Clearwater and lower Snake River regions of north-central Idaho". Journal of Archaeological Science: Reports 16: 258–265. doi:10.1016/j.jasrep.2017.09.030. ISSN 2352-409X. Bibcode: 2017JArSR..16..258C. https://www.sciencedirect.com/science/article/pii/S2352409X16304138.
- ↑ Grayson, Donald K.; Meltzer, David J. (2002). "Clovis Hunting and Large Mammal Extinction: A Critical Review of the Evidence". Journal of World Prehistory 16 (4): 313–359. doi:10.1023/A:1022912030020. http://link.springer.com/10.1023/A:1022912030020.
- ↑ Cannon, Michael D.; Meltzer, David J. (2004-10-01). "Early Paleoindian foraging: examining the faunal evidence for large mammal specialization and regional variability in prey choice". Quaternary Science Reviews 23 (18): 1955–1987. doi:10.1016/j.quascirev.2004.03.011. ISSN 0277-3791. Bibcode: 2004QSRv...23.1955C. https://www.sciencedirect.com/science/article/pii/S0277379104001052.
- ↑ Reid, Kenneth C. (2017-07-20). "Idaho beginnings: A review of the evidence". Quaternary International. After Anzick: Reconciling New Genomic Data and Models with the Archaeological Evidence for Peopling of the Americas 444: 72–82. doi:10.1016/j.quaint.2017.03.040. ISSN 1040-6182. Bibcode: 2017QuInt.444...72R. https://www.sciencedirect.com/science/article/pii/S1040618216314148.
- ↑ Kenady, Stephen M.; Wilson, Michael C.; Schalk, Randall F.; Mierendorf, Robert R. (March 2011). "Late Pleistocene butchered Bison antiquus from Ayer Pond, Orcas Island, Pacific Northwest: Age confirmation and taphonomy" (in en). Quaternary International 233 (2): 130–141. doi:10.1016/j.quaint.2010.04.013. Bibcode: 2011QuInt.233..130K. https://linkinghub.elsevier.com/retrieve/pii/S1040618210001369.
- ↑ Geist, Valerius (1989), "Did Large Predators keep Humans out of North America?", in Clutton-Brock, Juliet, The Walking larder: patterns of domestication, pastoralism, and predation, Unwin Hyman, pp. 282–294, ISBN 0-0444-5013-3, http://www.cnr.uidaho.edu/wlf520/pdf/geist.pdf
- ↑ 236.0 236.1 Pigati, Jeffrey S.; Springer, Kathleen B.; Honke, Jeffrey S.; Wahl, David; Champagne, Marie R.; Zimmerman, Susan R. H.; Gray, Harrison J.; Santucci, Vincent L. et al. (2023-10-06). "Independent age estimates resolve the controversy of ancient human footprints at White Sands" (in en). Science 382 (6666): 73–75. doi:10.1126/science.adh5007. ISSN 0036-8075. PMID 37797035. Bibcode: 2023Sci...382...73P. https://www.science.org/doi/10.1126/science.adh5007.
- ↑ 237.0 237.1 237.2 Boëda, Eric; Gruhn, Ruth; Vialou, Agueda Vilhena; Aschero, Carlos; Vialou, Denis; Pino, Mario; Gluchy, Maria; Pérez, Antonio et al. (2021-01-02). "The Chiquihuite Cave, a Real Novelty? Observations about the Still-ignored South American Prehistory" (in en). PaleoAmerica 7 (1): 1–7. doi:10.1080/20555563.2020.1851500. ISSN 2055-5563. https://www.tandfonline.com/doi/full/10.1080/20555563.2020.1851500.
- ↑ 238.0 238.1 Pansani, Thais R.; Pobiner, Briana; Gueriau, Pierre; Thoury, Mathieu; Tafforeau, Paul; Baranger, Emmanuel; Vialou, Águeda V.; Vialou, Denis et al. (2023-07-12). "Evidence of artefacts made of giant sloth bones in central Brazil around the last glacial maximum" (in en). Proceedings of the Royal Society B: Biological Sciences 290 (2002). doi:10.1098/rspb.2023.0316. ISSN 0962-8452. PMID 37434527.
- ↑ 239.0 239.1 239.2 Gruhn, Ruth (2023-07-03). "An Anthropological Conception of the Initial Peopling of the Americas" (in en). PaleoAmerica 9 (3): 167–173. doi:10.1080/20555563.2023.2278948. ISSN 2055-5563. https://www.tandfonline.com/doi/full/10.1080/20555563.2023.2278948.
- ↑ Graf, Kelly E.; Buvit, Ian (2017-12-01). "Human Dispersal from Siberia to Beringia: Assessing a Beringian Standstill in Light of the Archaeological Evidence". Current Anthropology 58 (S17): S583–S603. doi:10.1086/693388. ISSN 0011-3204. https://www.journals.uchicago.edu/doi/full/10.1086/693388.
- ↑ Harington, C. R.; Morlan, Richard E. (2002). "Evidence for Human Modification of a Late Pleistocene Bison (Bison sp.) Bone from the Klondike District, Yukon Territory, Canada". Arctic 55 (2): 143–147. doi:10.14430/arctic698. ISSN 0004-0843.
- ↑ Holen, Steven R.; Harington, C. Richard; Holen, Kathleen A. (2017). "New Radiocarbon Ages on Percussion-Fractured and Flaked Proboscidean Limb Bones from Yukon, Canada". Arctic 70 (2): 141–150. doi:10.14430/arctic4645. ISSN 0004-0843.
- ↑ Goebel, Ted; Hoffecker, John F.; Graf, Kelly E.; Vachula, Richard S. (June 2022). "Archaeological reconnaissance at Lake E5 in the Brooks Range, Alaska and implications for the early human biomarker record of Beringia" (in en). Quaternary Science Reviews 286. doi:10.1016/j.quascirev.2022.107553. Bibcode: 2022QSRv..28607553G.
- ↑ Bourgeon, Lauriane; Burke, Ariane; Higham, Thomas (2017-01-06). "Earliest Human Presence in North America Dated to the Last Glacial Maximum: New Radiocarbon Dates from Bluefish Caves, Canada" (in en). PLOS ONE 12 (1). doi:10.1371/journal.pone.0169486. ISSN 1932-6203. PMID 28060931. Bibcode: 2017PLoSO..1269486B.
- ↑ Bourgeon, Lauriane (2021-06-01). "Revisiting the mammoth bone modifications from Bluefish Caves (YT, Canada)" (in en). Journal of Archaeological Science: Reports 37. doi:10.1016/j.jasrep.2021.102969. ISSN 2352-409X. Bibcode: 2021JArSR..37j2969B. https://www.sciencedirect.com/science/article/pii/S2352409X21001814.
- ↑ Ardelean, Ciprian F.; Becerra-Valdivia, Lorena; Pedersen, Mikkel Winther; Schwenninger, Jean-Luc; Oviatt, Charles G.; Macías-Quintero, Juan I.; Arroyo-Cabrales, Joaquin; Sikora, Martin et al. (2020-08-06). "Evidence of human occupation in Mexico around the Last Glacial Maximum.". Nature 584 (7819): 87–92. doi:10.1038/s41586-020-2509-0. ISSN 0028-0836. PMID 32699412. Bibcode: 2020Natur.584...87A. https://www.repository.cam.ac.uk/handle/1810/312474.
- ↑ Pichardo, M. (1997). "Valsequillo biostratigraphy: New evidence for Pre-Clovis date". Anthropologischer Anzeiger 55 (3/4): 233–246. doi:10.1127/anthranz/55/1997/233. ISSN 0003-5548.
- ↑ Gonzalez, Sofia; Huddart, David (2008). "The Late Pleistocene Human Occupation of Mexico". FUMDHAMentos VII. https://www.researchgate.net/publication/265490787.
- ↑ 249.0 249.1 Gruhn, Ruth (2020-08-06). "Evidence grows that peopling of the Americas began more than 20,000 years ago" (in en). Nature 584 (7819): 47–48. doi:10.1038/d41586-020-02137-3. ISSN 0028-0836. PMID 32699366. Bibcode: 2020Natur.584...47G. https://www.nature.com/articles/d41586-020-02137-3.
- ↑ 250.0 250.1 Rowe, Timothy B.; Stafford, Thomas W.; Fisher, Daniel C.; Enghild, Jan J.; Quigg, J. Michael; Ketcham, Richard A.; Sagebiel, J. Chris; Hanna, Romy et al. (2022). "Human Occupation of the North American Colorado Plateau ∼37,000 Years Ago". Frontiers in Ecology and Evolution 10. doi:10.3389/fevo.2022.903795. ISSN 2296-701X. Bibcode: 2022FrEEv..1003795R.
- ↑ Gruhn, Ruth (2022), Miotti, Laura; Salemme, Monica; Hermo, Darío, eds., "To the End of the World: Southern Patagonia in Models of the Initial Peopling of the Western Hemisphere" (in en), Archaeology of Piedra Museo Locality, The Latin American Studies Book Series (Cham: Springer International Publishing): pp. 449–456, doi:10.1007/978-3-030-92503-1_16, ISBN 978-3-030-92502-4, https://link.springer.com/10.1007/978-3-030-92503-1_16, retrieved 2023-12-11
- ↑ Karrow, P.F.; Morgan, G.S.; Portell, R. W.; Simons, E.; Auffenberg, K. (1996-12-31), "Middle Pleistocene (early Rancholabrean) vertebrates and associated marine and non-marine invertebrates from Oldsmar, Pinellas County, Florida", Palaeoecology and Palaeoenvironments of Late Cenozoic Mammals (University of Toronto Press): pp. 97–133, doi:10.3138/9781487574154-009, ISBN 978-1-4875-7415-4, https://www.degruyter.com/document/doi/10.3138/9781487574154-009/html, retrieved 2024-01-23
- ↑ Feranec, Robert S.; Hadly, Elizabeth A.; Blois, Jessica L.; Barnosky, Anthony D.; Paytan, Adina (2007). "Radiocarbon Dates from the Pleistocene Fossil Deposits of Samwel Cave, Shasta County, California, USA". Radiocarbon 49 (1): 117–121. doi:10.1017/S0033822200041941. Bibcode: 2007Radcb..49..117F.
- ↑ 254.0 254.1 Stuart, Anthony John (May 2015). "Late Quaternary megafaunal extinctions on the continents: a short review: LATE QUATERNARY MEGAFAUNAL EXTINCTIONS" (in en). Geological Journal 50 (3): 338–363. doi:10.1002/gj.2633. https://onlinelibrary.wiley.com/doi/10.1002/gj.2633.
- ↑ Haynes, Gary (2013-02-08). "Extinctions in North America's Late Glacial landscapes". Quaternary International. Peopling the last new worlds: the first colonisation of Sahul and the Americas 285: 89–98. doi:10.1016/j.quaint.2010.07.026. ISSN 1040-6182. Bibcode: 2013QuInt.285...89H. https://www.sciencedirect.com/science/article/pii/S104061821000296X.
- ↑ Mitchell, Kieren J.; Bover, Pere; Salis, Alexander T.; Mudge, Caitlin; Heiniger, Holly; Thompson, Mary; Hockett, Bryan; Weyrich, Laura S. et al. (November 2021). "Evidence for Pleistocene gene flow through the ice-free corridor from extinct horses and camels from Natural Trap Cave, Wyoming" (in en). Quaternary International 647-648: 71–80. doi:10.1016/j.quaint.2021.11.017.
- ↑ Broughton, Jack M.; Weitzel, Elic M. (21 December 2018). "Population reconstructions for humans and megafauna suggest mixed causes for North American Pleistocene extinctions" (in en). Nature Communications 9 (1): 5441. doi:10.1038/s41467-018-07897-1. ISSN 2041-1723. PMID 30575758. Bibcode: 2018NatCo...9.5441B. Refer Supplementary Table 1
- ↑ Stynder, Deano D.; Kupczik, Kornelius (September 2012). "Tooth Root Morphology in the Early Pliocene African Bear Agriotherium africanum (Mammalia, Carnivora, Ursidae) and its Implications for Feeding Ecology" (in en). Journal of Mammalian Evolution 20 (3): 227–237. doi:10.1007/s10914-012-9218-x. ISSN 1064-7554. http://link.springer.com/10.1007/s10914-012-9218-x.
- ↑ Stuart, Anthony J.; Lister, Adrian M. (2012-09-19). "Extinction chronology of the woolly rhinoceros Coelodonta antiquitatis in the context of late Quaternary megafaunal extinctions in northern Eurasia". Quaternary Science Reviews 51: 1–17. doi:10.1016/j.quascirev.2012.06.007. ISSN 0277-3791. Bibcode: 2012QSRv...51....1S. https://www.sciencedirect.com/science/article/pii/S0277379112002326.
- ↑ "Beringia: Lost World of the Ice Age (U.S. National Park Service)" (in en). https://www.nps.gov/articles/aps-v12-i2-c8.htm.
- ↑ Blinnikov, Mikhail S.; Gaglioti, Benjamin V.; Walker, Donald A.; Wooller, Matthew J.; Zazula, Grant D. (2011-10-01). "Pleistocene graminoid-dominated ecosystems in the Arctic" (in en). Quaternary Science Reviews 30 (21): 2906–2929. doi:10.1016/j.quascirev.2011.07.002. ISSN 0277-3791. Bibcode: 2011QSRv...30.2906B. https://www.sciencedirect.com/science/article/pii/S027737911100206X.
- ↑ McHorse, Brianna K.; Orcutt, John D.; Davis, Edward B. (2012-04-15). "The carnivoran fauna of Rancho La Brea: Average or aberrant?" (in en). Palaeogeography, Palaeoclimatology, Palaeoecology 329-330: 118–123. doi:10.1016/j.palaeo.2012.02.022. ISSN 0031-0182. Bibcode: 2012PPP...329..118M. https://www.sciencedirect.com/science/article/pii/S0031018212000958.
- ↑ Wang, Xiaoming; Martin, Larry (1993-01-01). "Late Pleistocene, paleoecology and large mammal taphonomy, Natural Trap Cave, Wyoming". National Geographic Research & Exploration 9: 422–435. https://www.researchgate.net/publication/267156684.
- ↑ Veitschegger, Kristof (2017-06-05). "The effect of body size evolution and ecology on encephalization in cave bears and extant relatives". BMC Evolutionary Biology 17 (1). doi:10.1186/s12862-017-0976-1. ISSN 1471-2148. PMID 28583080. Bibcode: 2017BMCEE..17..124V.
External links
Template:Ursidae extinct nav Wikidata ☰ Q2626037 entry
 |