Biology:Glyptotherium
| Glyptotherium | |
|---|---|

| |
| G. texanum, National Museum of Natural History | |
| Scientific classification | |
| Script error: No such module "Taxobox ranks".: | Animalia |
| Script error: No such module "Taxobox ranks".: | Chordata |
| Script error: No such module "Taxobox ranks".: | Mammalia |
| Script error: No such module "Taxobox ranks".: | Cingulata |
| Script error: No such module "Taxobox ranks".: | Chlamyphoridae |
| Script error: No such module "Taxobox ranks".: | †Glyptodontinae |
| Script error: No such module "Taxobox ranks".: | †Glyptotherium Osborn, 1903 |
| Type species | |
| †Glyptotherium texanum Osborn, 1903
| |
| Other Species | |
| |

| |
| Distribution of Glyptotherium (orange) compared to Glyptodon's (green). | |
| Synonyms | |
Synonyms of G. texanum
Synonyms of G. cylindricum
| |
Glyptotherium (from Greek for 'grooved or carved beast') is a genus of glyptodont (an extinct group of large, herbivorous armadillos) in the family Chlamyphoridae (a family of South American armadillos) that lived from the Early Pliocene, about 3.6 million years ago, to the Late Pleistocene, around 15,000 years ago. It had a wide distribution, living in the United States , Mexico, Guatemala, Costa Rica, Honduras, El Salvador, Panama, Venezuela, and Brazil . The genus was first described in 1903 by American paleontologist Henry Fairfield Osborn with the type species being, G. texanum, based on fossils that had been found in the Pliocene Blancan Beds in Llano Estacado, Texas , USA. Glyptotherium fossils have since been unearthed from many more fossil sites, from Florida to Colombia. Another species, G. cylindricum, was named in 1912 by fossil hunter Barnum Brown on the basis of a partial skeleton that had been unearthed from the Pleistocene deposits in Jalisco, Mexico. The two species differ in several aspects, including age, with G. texanum being from the older Early Pliocene to Early Pleistocene strata, whereas G. cylindricum is exclusive to the Late Pleistocene.
Glyptotherium was a large, quadrupedal (four-legged), herbivorous armadillo with an armored carapace (top shell) that was made of hundreds of interconnected osteoderms (structures in dermis composed of bone). Other pieces of armor covered the tail and cranium roof, the skull being tall with hypsodont (high-crowned) teeth. As for the postcranial anatomy, pelves fused to the carapace, an amalgamate vertebral column, short limbs, and small digits are characteristic of Glyptotherium and its relatives. Glyptotherium reached up to 2 meters (6.56 feet) long and 400 kilograms (880 pounds) in weight, making it one of the largest glyptodonts but not as large as its close relative Glyptodon or Doedicurus, the largest known glyptodont. Glyptotherium is morphologically and phylogenetically most similar to Glyptodon, however they differ in several ways. Glyptotherium is smaller on average, with a shorter carapace, a relatively longer tail, and a slender zygoma, or cheek bone.
Glyptodonts evolved first during the Eocene, but greatly diversified in the Miocene and Pliocene, largely in the Santacrucian sites of Argentina . However, their diversity diminished into the Pleistocene, though they peaked in size during this period. Glyptotherium is considered an example of North American megafauna, of which most have become extinct, and may have been wiped out by changing climate or human interference. Glyptotherium was primarily a grazer, but also had a mixed diet of fruits and other plants, that lived on open grasslands. The armor could protect the animal from predators, of which many coexisted with Glyptotherium during its existence, including the "saber-tooth cat" Smilodon, the "bone-crushing dog" Borophagus, and the "short-faced bears" (Tremarctinae).
History and phylogeny
Fossils attributable to Glyptotherium have been found as early as the 1870s, when civil engineers J. N. Cuatáparo and Santiago Ramírez collected a skull, nearly complete carapace, and associated postcranial skeleton of a glyptodont from a drainage canal near Tequixquiac, Mexico, the fossils coming from the Rancholabrean age of the Pleistocene.[1][2][3] This was the first discovery of a glyptodont in North America.[2][3] Cuatáparo and Ramírez named the fossils Glyptodon mexicanum in 1875, but the fossils have been lost.[1][4] Another species of Glyptodon from Mexico was described in 1889, G. nathoristi, by German paleontologists based on carapace remains from Pleistocene localities in Ejutla, Oaxaca.[5][6] Both of these species have since been synonymized with G. cylindricum.[2][3]
The first Glyptotherium fossils to be described from the United States were described in 1888 by paleontologist Edward Drinker Cope and consisted only of a single carapace osteoderm that had been collected from the Lower Pleistocene "Equus Beds" of Nueces County, Texas .[7][2] Cope named his osteoderm Glyptodon peltaliferus,[8] but Cope did not give the species a proper description that followed ICZN rules, making it a nomen nudum and it has since been synonymized with G. cylindricum.[2][3] The next year, Joseph Leidy named Glyptodon floridanus based on isolated carapace osteoderms and pieces of caudal armor, though some were also referred to G. peltaliferus,[9] that were collected from Pleistocene deposits in DeSoto County, Florida.[10][11] This species is now seen as a nomen vanum and considered a junior synonym of Glyptotherium cylindricum by a review of the genus by American paleontologists David Gillette and Clayton Ray (1981).[12][2]
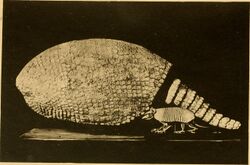
Glyptotherium itself was named in 1903 when fossils collected by an American Museum of Natural History expedition led by J. W. Gidley to the Early Pliocene strata from the Blanco Formation of Llano Estacado, Texas were described by Henry Fairfield Osborn as a new genus and species of glyptodont, Glyptotherium texanum.[7] The fossils were deposited at the AMNH and consist of a carapace and associated postcranial elements, one of the few G. texanum skeletons known.[7][2] The generic name Glyptotherium comes from the Greek roots glyph meaning "carved" or "grooved", after its relative Glyptodon, and therion meaning "beast", a commonly used suffix for prehistoric mammals. The species name of the type species, G. texanum, is after the holotype's discovery in Texas.[7]
Another find came in 1910 when, while traveling in Jalisco, Mexico, fossil hunter Barnum Brown collected a complete dorsal carapace and several additional fossils, including teeth, of a single individual from the Pleistocene strata of the area.[3][13] The specimen was sent to the American Museum of Natural History as well, where it was described by Brown in 1912 as a new genus and species of glyptodont, Brachyostracon cylindricum.[13] The species name cylindricum meaning "cylindrical" is after the cylindroid anatomy of the premolars in the holotype of G. cylindricum.[13] Brown also recombined Glyptodon mexicanum into Brachyostracon mexicanum.[13] Brachyostracon is now seen synonymous with Glyptotherium on the genus level, but G. cylindricum is a valid species.[2][14] In 1923, Oliver Perry Hay named a new species of Glyptodon, G. rivipacis, based on the fossils described by Leidy from DeSoto County, Florida.[15] This species is now seen as a nomen nudum and synonymous with Glyptotherium cylindricum.[2][16] Hay also described well preserved fossils, including skull elements and teeth, that were collected from Rancholabrean strata in Wolf City and Sinton, Texas that were referred to Cope's Glyptodon peltaliferus.[14] These fossils have since been referred to G. cylindricum.[2][14]
A third genus of North American glyptodont, Boreostracon floridanum, was established by George Gaylord Simpson in 1929 based on several isolated specimens unearthed by the AMNH from Rancholabrean age localities in Florida, the holotype specimen being the rear portion of a carapace recovered from Seminole Field locality in Pinellas County, Florida.[12][17] Simpson referred all of the fossils previously described from Florida to B. floridanum and believed that all of the glyptodont fossils unearthed from North America were not of Glyptodon.[17] However, Simpson chose not to designate a new genus or species for Glyptodon peltaliferus, but he still believed that they were from a separate form of glyptodont.[17] Boreostracon floridanum has been synonymized with Glyptotherium cylindricum.[2]
Research into North American glyptodonts diminished after the research of Gidley, Hay, Simpson, and others, but some paleontologists still incorrectly referred fossils from the continent to Glyptodon.[14]
In 1927, many Early Pleistocene age fossils were collected by the University of Oklahoma from a locality in Frederick, Oklahoma, including several fragmentary fossils of glyptodonts, equids, gomphotheres, and camelids.[18] The glyptodont fossils were originally referred to Glyptodon in 1928,[19][20] but were not properly described until 1953.[18] They were described as a new genus and species, Xenoglyptodon fredericensis, in 1953 on the basis of a partial lower jaw and several teeth.[18] The species is now considered a junior synonym of Glyptotherium texanum.[2][14] After all of these genera were named, a great reassessment was not conducted until 1981, David Gillette and Clayton Ray published a monograph on North American glyptodonts. In their monograph, they recombined all previously named genera and species into Glyptotherium, synonymized some species, and also researched the genus' ecology, anatomy, and distribution.[14] However, G. arizonae, G. floridanum, and G. mexicanum were kept as valid species,[14] all of which were later synonymized with G. texanum and G. cylindricum after the discovery of more complete skeletons that proved their synonymy.[2][21] After later review, the former species was declared a junior synonym of G. texanum[2][21] and the latter two synonyms of G. cylindricum.[2]
From the 2000s to the 2020s, hundreds of additional fossils were referred to the genus from Central America and Brazil.[22][23] These include fossils previously referred to Glyptodon and Hoplophorus, as many fossils were hastily assigned to both by 19th century paleontologists.[22][24] One of the specimens reassigned to Glyptotherium, an isolated dorsal carapace osteoderm, was collected from Pleistocene age carbonate caves in Lagoa Santa, Minas Gerais, Brazil by Friedrich von Sellow during the early 1800s.[22] It was later described as a new species of Hoplophorus, H. meyeri, in 1845 by Danish paleontologist Peter Wilhelm Lund.[25][24][22] Lund incorrectly named the taxon however, making it a nomen nudum.[22] The osteoderm was referred to Glyptotherium in 2010, making it the first known Glyptotherium specimen.[22] In 2022, a host of fossils of Glyptotherium cylindricum including skulls, some preserving pathologies caused by humans, were described that had been collected from several sites in Falcón, northern Venezuela that dated to the Late Pleistocene.[26]

Phylogeny
Glyptotherium is a genus in the subfamily Glyptodontinae, an extinct clade of large, thoroughly armored armadillos that first evolved in the Late Eocene (ca. 33.5 mya) and went extinct in the Late Pleistocene-Early Holocene extinctions.[27] Glyptodontinae was classified in its own family or even superfamily until in 2016, when ancient DNA was extracted from the carapace of a 12,000 year old Doedicurus (a large, mace-clubbed glyptodont) specimen, and a nearly complete mitochondrial genome was reconstructed (76x coverage). Comparisons with the DNA of modern armadillos revealed that glyptodonts diverged from tolypeutine and chlamyphorine armadillos approximately 33.5 million years ago in the late Eocene.[27][28] This prompted moving them from their own family, Glyptodontidae, within the extant Chlamyphoridae as a subfamily, renamed Glyptodontinae.[28] Following this genetic data and the fossil record, glyptodonts rapidly evolved their characteristic anatomy and enormous sizes (also known as gigantism), possibly due to the cooling of temperatures, dryer climates, expansion of savannahs, and the size of carnivores like Arctotherium and Smilodon.[27] Chylamyphoridae is a group in the order Cingulata, which includes all extant armadillos in addition to other fossil groups like Pachyarmatheriidae and Pampatheridae. Cingulata is itself within the basal mammal group Xenarthra, which includes an array of American mammal groups like Vermilingua (anteaters) and Folivora (sloths and ground sloths) in the order Pilosa. The following phylogenetic analysis was conducted by Frédéric Delsuc and colleagues in 2016 and represents the phylogeny of Cingulata using ancient DNA from Doedicurus to determine the position of it and other Glyptodonts:[27][29]
| Cingulata |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

The internal phylogeny of Glyptodontinae is convoluted and in a flux, with many species and families erected on the basis of fragmentary or undiagnostic material that lacks comprehensive review.[30][24][2] It is usually considered its own family, but DNA analyses have reduced it to a subfamily with tribes instead of subfamilies. One tribe, Glyptodontini (typically labeled Glyptodontinae) is a group of younger, larger glyptodonts that evolved in the Middle Miocene (ca. 13 mya) with Boreostemma,[31] but split into 2 genera, Glyptodon in the south and Glyptotherium in the north,[2] though Glyptodon also lived in northern South America in Colombia.[2][32] Glyptodontini is often recovered as more basal to most other glyptodonts like Doedicurus, Hoplophorus, and Panochthus. Glyptodontini is distinguishable from other groups for example in that it has large, conical tubercular osteoderms absent or only present on the caudal (tailward) notch on the posterior end of the carapace and different ornamentation of the armor on the carapace than the tail.[33] The sister taxon, or closest relative(s) of another taxon, to Glyptotherium is the genus Glyptodon, which evolved in the Middle Pleistocene in Argentina.[33][30] Glyptotherium is nearly identical to Glyptodon in many aspects, so much so that the first fossils of Glyptotherium to be described were misidentified as those of Glyptodon.[14][1]
When Glyptotherium was first described by Henry Fairfield Osborn in 1903, he placed it at the family level Glyptodontidae and although similar to Glyptodon, Osborn stated that it was closer in appearance and classification to Panochthus and Neosclerocalyptus (then Sclerocalyptus).[7] Barnum Brown believed that his genus Brachyostracon and its 2 species, B. cylindricum and B. mexicanum, were in their own family of glyptodonts based on the elongation of the skull of the latter species and the width of the carapace compared to its length.[13] Brown did not coin any new name for this family however, and instead classified the genus within Sclerocalyptidae along with South American glyptodonts like Panochthus, Neosclerocalyptus, and Plohophorus.[13] George Gaylord Simpson classified his genus Boreostracon as a close relative of Glyptodon, but still believed that there were multiple North American glyptodont genera.[17] Xenoglyptodon was placed as a glyptodont close to the other North American genera by Meade (1953), but he did not state its relation to South American genera.[18]Below is the phylogenetic analysis conducted by Cuadrelli et al., 2020 of Glyptodontinae, with Glyptodontidae as a family instead of subfamily, that focuses on advanced Glyptodonts:[33]
| Chlamyphoridae |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Description

Like its living relative, the armadillo, Glyptotherium had a shell that covered its entire torso, with smaller armor also covering the skull roof of the head, similar to a turtle. However, unlike the carapace (top shell) of a turtle, the Glyptotherium shell was made up of hundreds of small hexagonal scales, with Glyptotherium preserving up to 1800 osteoderms (bony structures in dermis) or more in each individual.[34] The axial skeleton of glyptodonts show extensive fusion in the vertebral column and the pelvis (hipbone) is fused to the carapace, making the pelvis entirely immobile.[14] Glyptotherium was very graviportal and had short limbs that are very similar to those in other glyptodonts. The large tails of glyptodonts likely served as a counterbalance to the rest of the body and Glyptotherium's caudal armor ended in a blunt tube that was composed of 2-3 fused tubes,[2] in contrast to those of South American mace-tailed glyptodonts.[35] The digits of Glyptotherium are very stout and adapted for weight-bearing, though some preserve large claw sheaths that had an intermediate morphology between claws and hooves.[14] During the Pleistocene, the glyptodonts diminished in phylogenetic diversity but increased in size.[26][2] Glyptotherium weights and sizes vary, but G. texanum was smaller and lighter than the later species, G. cylindricum. One specimen of G. cylindricum was estimated at 350-380 kilograms, compared to 457 kg of its relative Glyptodon reticulatus.[26] The largest estimates of Glyptotherium's mass are based on adult specimens from Brazil and Arizona, with one estimate of a G. cylindricum specimen placing it at 710 kilograms (1,570 lb) and a G. texanum individual at 1,165 kilograms (2,568 lb).[36] One specimen of G. cylindricum, AMNH 15548, preserved a carapace length of 1690 centimeters compared to just 1400 cm for MSM P4464, a G. texanum specimen.[2]

Glyptotherium texanum differs from G. cylindricum in the configuration of the external sculpturing of symmetrical, hexagonal osteoderms in the dorsal carapace. In the osteoderms of adult G. texanum, the central figures of the osteoderms are flat or weakly convex and very large, while those of G. cylindricum are flat or concave and much smaller.[2][37] In both species, the central figures of the osteoderms get larger towards the margins of the carapace.[2][21] Although G. texanum was believed to be larger than G. cylindricum, the genus is believed to have been uniformly large throughout the Pliocene and Pleistocene.[2]
Skull and mandible
Glyptodont dentition has all hypsodont (high crowned teeth adapted for grazing) molariforms, cheek teeth, which are some of the most hypsodont known from terrestrial mammals.[38] Glyptodont skulls have several unique features; the maxilla and palatine are enlarged vertically to make space for the molariforms, while the braincase is brachycephalic, short and flat.[39] Dermal armor was not restricted to the carapace and tail, as the skull roof was protected by a "cephalic shield" made of osteoderms.[26][40] Some paleontologists have proposed that Glyptotherium and some glyptodonts also had a proboscis or large snout similar to those in elephants and tapirs,[41] but few have accepted this hypothesis.[2][26] Only one complete skull is known from Glyptotherium texanum, while relatives like Glyptodon and Neosclerocalyptus are known from many skulls, giving a limited perspective on its anatomy.[42][43] Glyptotherium's zygoma are narrow, slender, almost parallel, and close to the sagittal plane in frontal view; in Glyptodon, this structure is broader, robust, divergent rather than parallel and more laterally placed. Glyptotherium and other glyptodonts preserve large nasal passages and sinuses that may have had nostrils adapted to breath in the cold arid climates of the Americas during the Pleistocene.[44] In turn, the infraorbital foramina are narrow and not visible in anterior view in Glyptotherium, but in Glyptodon they are broad and clearly visible in anterior view. In lateral view, the dorso-ventral height between the skull roof and the palatal plane in Glyptodon decreases anteriorly, contrary to Glyptotherium; the nasal tip is in a lower plane with respect to the zygomatic arch in Glyptodon, but in Glyptotherium is higher than the zygomatic arch plane. In Glyptotherium, the occlusal lateral profile is slightly curved, whereas it is strongly curved in Glyptodon. In Glyptodon, the 1st molariform (abbreviated as mf1) is distinctly trilobate (three-lobed) both lingually and labially, nearly as trilobate as the mf2; on the contrary, Glyptotherium shows a very low trilobation of mf1, which is elliptical in cross section, the mf2 is weakly trilobate, and the mf3 is trilobate. In both genera, the mf4 to mf8 are fully trilobate and serially identical.[2]
The mandibles of Glyptotherium and Glyptodon are very similar, but Glyptotherium's mandible is smaller by about 10%. The angle between the occlusal plane and the anterior margin of the ascending ramus is approximately 60 in Glyptotherium, while it is 65° in Glyptodon. The ventral margin of the horizontal ramus is more concave in Glyptodon than in Glyptotherium. The symphysis area is extended greatly in Glyptotherium antero-posteriorily compared to Glyptodon. The mf1 is ellipsoidal in Glyptotherium and the mf2 is submolariform, while in Glyptodon both teeth are trilobate.[2]
Carapace and osteoderms
File:Osteoderms of different cingulates - Lima et al 2018.tif Glyptotherium, and all other glyptodonts, had a large dorsal carapace covering much of the dorsum that was made up of interconnected osteoderms. The carapace of Glyptotherium was shorter than that of its relative Glyptodon, but much more elongated than that of Boreostemma. The high point of the carapace was at the center of the midline, while Glyptodon's was slightly displaced. Glyptotherium's carapace was strongly arched, with a convex pre-iliac and concave post-iliac, giving it a saddle-like overhang over the tail.[2][45] In Glyptodon the top-bottom height of the carapace represents 60% of its total length, whereas in Glyptotherium it is taller at ca. 70%. The ventral margins of the carapace in Glyptotherium is much more rectangular and less convex than in Glyptodon. In Glyptotherium, the osteoderms in the antero-lateral areas of the carapace are less ankylosed than in Glyptodon, suggesting that the antero-lateral carapace regions of the former had more flexibility. The osteoderms of the caudal aperture are more conical in Glyptodon and more rounded in Glyptotherium, though in the latter the anatomy of the caudal aperture osteoderms varies by sex[21] while in Glyptodon it varies by age.[46] Although frequently used to differentiate the two taxa, Glyptotherium and Glyptodon have very similar osteoderm morphologies that differ only in several areas. Both genera have very thick osteoderms compared to those of many South American glyptodonts like Hoplophorus and Neosclerocalyptus, but Glyptotherium always preserve a "rossette" pattern, where the osteoderm's central figure is surrounded by a row of peripheral figures. Some Glyptodon specimens preserve these "rossettes", but others lack them. The central and radial sulci are deeper and broader in Glyptodon (ca. 4–6 mm) than in Glyptotherium (ca. 1–2.4 mm).[2][21] Notably, Glyptotherium osteoderms preserve small gaps for hair follicles in the sulci that indicate that Glyptotherium had a "fuzzy" carapace with fur coming out. The number of follicles varies between ages and the area of the carapace, with juveniles having more follicles than adults, and fewer follicles are known from the lateral, caudal, and rear portions of the carapace.[47][21]
Caudal rings

Glyptotherium is a glyptodont, meaning its caudal armor is made up of a series of caudal rings ending in a short caudal tube, in contrast to the mace-like ends in other glyptodonts, but the morphology differs between Glyptotherium, Glyptodon, and Boreostemma. Overall, Boreostemma preserves a more similar caudal armor to Glyptotherium than to Glyptodon.[45] The caudal armor is longer in Glyptotherium than in Glyptodon, with one specimen of G. texanum (UMMP 34 826) preserving a meter long set of caudal armor. In Glyptotherium, the caudal armor length represents circa 50% of the dorsal carapace's total length, whereas in Glyptodon, this value is lower at around 30-40%. Glyptodon has 8–9 complete caudal rings plus one caudal tube, but Glyptotherium preserves 1 incomplete caudal ring in addition to the 8–9 complete caudal rings and caudal tube. In both genera, each caudal ring is composed of two or three transverse rows of ankylosed osteoderms, where the distalmost row of osteoderms shows a more or less developed conical morphology. In Glyptotherium, in some specimens (e.g., AMNH 95,737) a low number of conical osteoderms (generally two). This is different from Glyptodon, in which most osteoderms of the distal row (up to 12) present a clear conical morphology. The terminal caudal tube is shorter in Glyptodon. In Glyptotherium, the terminal tube is composed of 2–3 ankylosed rings, whereas, in Glyptodon, it has only two ankylosed rings. In Glyptotherium, this caudal tube represents ca. 20% of the total length of the caudal armor, whereas in Glyptodon, this structure represents 13% of the total length.[2]
Paleobiology
Posture
Several interpretations of glyptodont posture have been made,[48] initially by British paleontologist Richard Owen in 1841 using comparative anatomy.[49] Owen theorized that the phalanges were weight-bearing due to their short and broad physiology, in addition to the evidence provided in the postcranial skeleton.[49] It was also proposed that an upright posture was possible for glyptodonts, first by Sénéchal (1865) who stated that the tail could be an equilibrium for the front half of the body as well as a method of supporting the legs.[50][48] Linear measurements were later taken which provided insight into this hypothesis, finding that bipedalism would be possible.[51][52] The patellar articulation with the femur suggests rotation of the crus during knee extension and potentially even knee-locking were feasible.[53]
Feeding and diet

Glyptotherium is traditionally thought to consume wet, riparian herbs.[54] The genus was mainly a grazer but also had a mixed diet of C3 and C4 plants based on isotope analyses of dental specimens recovered from the Late Pleistocene Cedral locality in San Luis Potosí, México.[55] The locality preserves C4 plants from the families Poacea, Amaranthacea and Quenopodiacea,[56] meaning that they were possible food sources for Glyptotherium.[55] Cedral specifically was an area with hot springs and open grasslands next to them, suggesting that Glyptotherium fed in grasslands near water sources, like the feeding habits of modern capybaras.[41][55] Additional isotopic analysis of Glyptotherium and the giant ground sloth Eremotherium found the two to have similar isotopic levels to the extant amphibious Hippopotamus, indicating that they were semi-aquatic herbivores that fed on aquatic plants. Additional studies of dental specimens from eastern Brazil suggest that the Glyptotherium were grazers in moist, lowland tropical to subtropical habitats along rivers or water sources, supporting the semi-aquatic Glyptotherium hypothesis.[36][23][57] Additional isotopic evidence from Brazil suggests that fruits were also part of Glyptotherium diets, though only around 20% total.[58] Glyptotherium and all other glyptodonts had hypsodont teeth, high-crowned teeth with rough, flat surfaces adapted for grinding and crushing, that were adapted to break down gritty, fibrous material like grasses.[59][41] This diet for Glyptotherium contrasts with those surmised for their relatives Pampatheres, which have been considered insectivores or grazers.[55][60] Like most other xenarthrans, glyptodonts had lower energy requirements than most other mammals. They could survive with lower intake rates than other herbivores with similar mass.[61]
Digging abilities
Many extant species of armadillo have digging capabilities, with large claws adapted for scraping dirt to make burrows or forage for food underground.[62][63] Also, much of their diet consists of insects and other invertebrates that live underground,[64] in contrast to the herbivorous diets of Glyptotherium.[65] Being from the armadillo family, glyptodont fossorial capabilities have been researched on several occasions.[48] Owen (1841) opposed this idea, though pushback came from Nodot (1856) and Sénéchal (1865) who believed digging was possible for the genus.[48][49] However, the evolution of a rigid carapace as opposed to a flexible one in extant armadillos as well as a weakly developed deltoid crest on the humerus (upper arm bone) provided evidence against fossorial hypotheses. The elbow had a great range of movement, as with digging cingulates, but this is more likely to be due to size adaptations.[66][48]
Ontogeny
Juvenile and adult specimens of Glyptotherium texanum found in Blancan localities in Arizona preserve a nearly complete growth series, one of the few known in glyptodonts.[21] The teeth of Glyptotherium preserve hypsodonty, where the teeth do not stop growing.[21] In the juveniles, fully articulated osteoderm-to-osteoderm contact is already known, even at their age.[21] Growth of the osteoderms continued from juveniles to sub-adults and ceased when osteoderms became ankylosed (fused).[17][21] The lateral profile of juvenile carapaces are gently convex into the high-arched carapaces of adults. Another ontogenetic change is in the sulci and peripheral figures of the osteoderms; the central figures are the largest relative to the sulci in newborns and juveniles, but this ratio becomes lower in adults. In G. cylindricum, however, the osteoderms grow much faster and the sulci are much smaller. The osteoderms are also relatively thicker in juvenile Glyptotherium individuals compared to adults.[21]
Sexual dimorphism
Individuals of Glyptotherium texanum found in Blancan localities in Arizona preserve sexual dimorphism between male and female individuals. The caudal aperture, large conical osteoderms that protect the base of the tail, of males and females differ in that the marginal osteoderms of males are much more conical and convex than those of females. Even in the carapaces of newborn Glyptotherium, the marginal osteoderms are either conical or flat, which enables their sex to be determined.[21]
Osteoderms and protection

Osteoderms of Glyptotherium are made of a cancellous trabecular core in between two compact layers. Each hexagonal osteoderm is joined to the adjacent osteoderms with sutures, creating a large, robust carapace similar to those in modern animals. However, the carapaces of glyptodonts like Glyptotherium were far less flexible than those of modern armadillos.[67] The trabecular core was made up of struts used for support with an average thickness of 0.25 mm, these struts making up the central support of the osteoderm. Mechanical analyses revealed that smaller load areas, representing sharper objects, cause higher stresses than those caused by large, blunt objects. This can be understood as the natural structure evolved to withstand blunt impact from large objects such as tail-clubs and not as protection against sharp objects such as teeth.[37] This presents further evidence for the theory that glyptodonts used their carapaces for intraspecific combat using tail clubs as well as defense.[37]
Disease and pathologies
A partial postcranial skeleton of Glyptotherium cylindricum found in northeastern Brazil that included partial limbs preserved 3 different types of arthritis: one type of inflammatory arthritis (spondyloarthropathy), one type of crystalline arthritis (calcium pyrophosphate deposition disease) and one proliferative arthritis (osteoarthritis). Spondyloarthropathy was the most prevalent, with bone erosion on the right ulna, right and left radii, a left femur and both right and left tibiae-fibulae and is associated with bone sclerosis on the right ulna and radius. Calcium pyrophosphate deposition disease is present on the articular surface of the left patella and osteoarthritis is represented by osteophytes on some of the left hindlimb.[68] On several osteoderms that were also found in Brazil, ectoparasitism, lesions, and growths were found, some of these infections were likely caused by fleas.[69]
Paleoecology
Glyptotherium was primarily a grazer in forested grasslands and arboreal savannahs, though they may have preferred grasslands near water sources based on fossils localities from Mexico.[55] Due to their wide distribution, Glyptotherium's paleoecology may have varied across regions and its 2 species.[23][55]

In North America, Glyptotherium is known from 2 different species that lived in 3 different intervals, the Blancan, Irvingtonian, and Rancholabrean, across several US states and Mexico.[2] However, Glyptotherium has not been found in Pacific drainages in the United States or west of the Colorado River,[2] being instead present largely in the Atlantic Plain and Interior Plains.[70] During the Blancan, Glyptotherium texanum coexisted with many endemic genera from North America, as Beringia had not yet formed. Because of this, the fauna of the Blancan starkly contrasted with the fauna of the proceeding Pleistocene. The Blancan age strata of western Texas, New Mexico, and Arizona preserves the gomphothere proboscideans Stegomastodon and Cuvieronius, equids represented by grazers like Nannipus and Equus. Other xenarthrans are also known, like the megalonychid ground sloth Megalonyx and the mylodontid Paramylodon. Fossils of small mammals have also been unearthed, like the insectivores Hesperoscalops and Sorex. A giant fossil ground squirrel, Paenemarmota, is also known from the Blancan.[71] The carnivores include the unusual "bone-crushing" dog relative Borophagus and the "running hyena" Chasmaporthetes,[72] in addition to the "saber-toothed" cat Smilodon gracilis.[73] Some isolated bird fossils have also been found consisting of vultures, falcons, and possibly corvids.[72][74][75] Fossils from Guatemala were found from high altitudes, showing that Glyptotherium was highly adaptable and could live in a variation of environments.[76] This is supported by the generalist diet of Glyptotherium and fossil's discoveries in tropical, subtropical, forested, and even semi-aquatic environments. In the Mexican state of Yucatan, fossils of Glyptotherium and the ground sloth Paramylodon are known from water-rich areas like riparian forests and swamps as opposed to open grasslands.[77]
In the Brazilian Intertropical Region (BIR) in eastern Brazil, Glyptotherium was a mixed grazer in arboreal savannahs, tropical grasslands, and other grassy areas near water sources. Large, mesoherbivore mammals in the BIR were widespread and diverse, including the cow-like toxodontids Toxodon platensis and Piauhytherium, the macraucheniid litoptern Xenorhinotherium and equids such as Hippidion principale and Equus neogaeus. Toxodontids were large mixed feeders as well and lived in forested areas, while the equids were nearly entirely grazers. Other xenarthran fossils are present in the area as well from several different families, like the giant megatheriid ground sloth Eremotherium, the scelidotheriids Catonyx and Valgipes, the mylodontids Glossotherium, Ocnotherium, and Mylodonopsis. Smaller ground sloths such as the megalonychids Ahytherium and Australonyx and the nothrotheriid Nothrotherium have also been found in the area. Eremotherium was a generalist, while Nothrotherium was a specialist for trees in low density forests, and Valgipes was an intermediate of the two that lived in arboreal savannahs. Other glyptodonts and cingulates like the grazing glyptodont Panochthus and the omnivorous pampatheres Pampatherium and Holmesina were present in the open grasslands. A proboscidean species has also been found in the BIR, Notiomastodon platensis, was also present and was a mixed grazer on the open grasslands. Carnivores included some of the largest known mammalian carnivores, like the giant felid Smilodon populator and the bear Arctotherium wingei.[56][57] Several extant taxa are also known from the BIR, like guanacos, giant anteaters, collared peccaries, and striped hog-nosed skunks.[78] Two crab-eating types of extant mammals are also known from the BIR, the crab-eating raccoon and the crab-eating fox, indicating that crabs were also present in the region.[78] The environment of the BIR is unclear, as there were both several species that were grazers, but the precede of the arboreal fossil monkeys Protopithecus and Caipora in the area causes confusion over the area's paleoenvironment. Most of Brazil was thought to have been covered in open tropical cerrado vegetation during the Late Pleistocene, but if Protopithecus and Caipora were arboreal, their presence suggests that the region may have supported a dense closed forest during the Late Pleistocene.[78][79] It is possible that the region alternated between dry open savanna and closed wet forest throughout the climate change of the Late Pleistocene.[80]
Great American Interchange
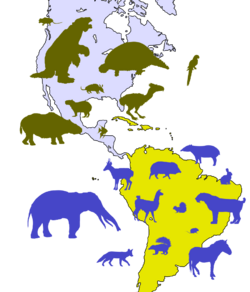
South America, the continent where glyptodonts originated, was isolated after the breakup of the landmass Gondwana at the end of the Mesozoic era.[81] This period of separation from the rest of the Earth's continents led to an age of unique mammalian evolution, with the dominance of groups such as marsupials, xenarthrans, and notoungulates in contrast to the North American mammal fauna. Marsupials likely got to South America prior to its separation from the rest of Gondwana in the Late Cretaceous or Paleogene, although the origins of mammalian orders like Xenarthra and Notoungulata ended up on the continent remains a mystery.[82] There were several movements of outside mammals to South America prior to the formation of the Isthmus of Panama, such as with primates and rodents which may have rafted to the continent from Africa and the movement of bats via flight.[83][84] As for the fauna of North America, contemporary groups like canids, felids, ursids, tapirids, antilocaprids, and equids populated the region in addition to extinct families like gomphotheres, amphicyonids, and mammutids.[85][81] The Great American Interchange did not enter its biggest stage until the Isthmus of Panama formed 2.7 million years ago during the Blancan stage of the Pliocene.[86][87][88] This intensified movement of glyptodonts, ground sloths, capybaras, pampatheres, terror birds, and marsupials to North America via the Central America route and a reverse migration of ungulates, proboscideans, felids, canids, and many other megafauna groups to South America.[89] The period following the Isthmus' foundation witnessed the extinction or extirpation of many groups, including the South American terror birds, toxodonts, macraucheniids, pampatheres, ground sloths, and glyptodonts.[90][91]
Glyptotherium itself was part of this interchange, evolving in the Blancan of the Mexico & the USA after the formation of the Isthmus and its immigration.[2][89] The geographic isolation of Glyptotherium in combination to adaptations to the different environments of North America led to Glyptotherium breaking off from other glyptodontines.[2]
Though G. texanum evolved in North American into G. cylindricum in the Rancholabrean, it emigrated southwards to Central and parts of northern South America circa 15,000-20,000 BP.[26][22][92] This is also connected to ecological segregation, with mountain barriers in Colombia possibly separating Glyptodon and Glyptotherium.[32] Subsequently, Glyptodon lived primarily in Andean and coastal sites, with Glyptotherium being known from grassland and lightly forested lowland deposits near aquatic areas, motivating its dispersal to the tropical regions of Venezuela and eastern Brazil.[88][23][93] The re-entrance of a group to South America from North America has also been observed in the related cingulate family Pampatheriidae,[87] possibly aided by wide coastal lowlands uncovered during glacial periods on the Caribbean and Atlantic coasts, allowing migration between Florida, Mexico, Central America, and/or northern South America.[88][94]

Predation
Smilodon may have occasionally preyed upon Glyptotherium, based on a skull from one Glyptotherium texanum individual recovered from Pleistocene deposits in Arizona bearing the distinctive elliptical puncture marks that best match those of the machairodont cat, indicating that the predator successfully bit into the skull through the armored cephalic shield.[95] The Glyptotherium in question was a juvenile, with a still-developing head shield, making it far more vulnerable to the cat's attack.[96] Although originally theorized by George Brandes to be possible in 1900,[97] Smilodon canines could not pierce the thick carapace osteoderms of glyptodonts.[98] Brandes hypothesized that the evolution of thick glyptodont armor and long machairodont canines was an example of coevolution,[97] but Birger Bohlin argued in 1940 that the canines were far too fragile to do damage against glyptodont armor.[98][46] However, the evolution of accessory protection structures may have been in response to the arrival of Smilodon and Arctotherium in the Ensenadan.[46]
In 2017, a right ulna of an adult Glyptotherium that had been collected from Late Pleistocene strata in Rio Grande do Norte State, Brazil was described that bore several gnawing traces from a new ichnospecies of Machichnus, M. fatimae, that may have been caused by a juvenile of the canid species Protocyon troglodytes or an adult individual of Cerdocyon thous.[99]
Relationship with humans

The first report of possible human consumption or interaction with Glyptotherium or its fossils came in 1958, where several osteoderms that were possibly consumed by humans were described from the Clovis site in Lewisville, Texas.[100][101] This idea of human consumption has little evidence to back it, however.[26] In 2022, a host of fossils of Glyptotherium cylindricum including skulls were described that had been collected from several sites in Falcón, northern Venezuela that dated to the Late Pleistocene.[26] These discoveries were notable not only because they preserved skulls, but four of them exhibited breakages in the fronto-parietal region, a pattern in all of the skulls. Visual and CT evidence indicates that these were likely caused by a mechanical effect by direct percussion, most likely a blow with a stone chopper or club, which caused the bones in the region to fragment into the soft internal tissues of the skull. Despite the fact that the skulls were complete and showed no signs of taphonomic distortion or transport, they often lacked their jaws. The jaws may have been removed for "hunters" to access and consume masticatory muscles and tongue. The coexistence of early hunter-gatherer humans and glyptodonts in South America was first hypothesized in 1881 based on fossil discoveries from the Argentine Pampas,[102] and many fossil discoveries from the Late Pleistocene have been unearthed since that exhibit human predation on glyptodonts. During this period, a wide array of Xenarthrans inhabited the Pampas were hunted by humans, with evidence demonstrating that the small (300-450 kg) glyptodont Neosclerocalyptus,[103] the armadillo Eutatus, and the gigantic (2 ton) glyptodont Doedicurus, the largest glyptodont known, were hunted.[104] The only other record of human predation from outside the Pampas was a partial carapace, found also in Venezuela, that was eviscerated by humans. The discoveries in the localities in Falcón showed the first signs of human hunting on the skulls of glyptodonts, but Glyptotherium also was more defenseless than glyptodonts like Doedicurus.[26][105]
Distribution
Glyptotherium is the only known North American glyptodont and is known from several regions of the continent from different periods. During the Blancan stage of the Early Pliocene, Glyptotherium texanum inhabited only central Mexico based on the discovery of a single osteoderm of Glyptotherium texanum from the early Pliocene strata of Guanajuato, central Mexico, dating to approx. 3.6 million years ago.[2][106] In the Blancan-Irvingtonian stages of the Early Pleistocene, G. texanum fossils are known from most of Mexico as well as the U.S. states of Arizona, Texas , Oklahoma, Florida, and possibly South Carolina.[2][106] In the Rancholabrean of the Late Pleistocene, G. cylindricum evolved from G. texanum and its fossils have been unearthed from northern Venezuela, eastern Brazil , Central America, Mexico, and the U.S. states of Texas, Louisiana, Florida, and South Carolina.[2][106][107][54] Fossils from Glyptotherium from the Early Pliocene have not been found in Central America, but it is likely that G. texanum inhabited the area during the Great American Biotic Interchange. Glyptodont fossils from the middle-late Irvingtonian are not known from the USA, creating a "glyptodont gap" in the US fossil record.[2] However, Glyptotherium is recorded during this "pause" in Central America, suggesting a possible retraction of Glyptotherium to southern areas during glacial times.[92]

Glyptotherium fossils have been collected from Central America in Guatemala,[108] Costa Rica,[109] Honduras,[110] El Salvador,[111] and Panama.[112] The fossils from Central America are usually isolated and fragmentary, with the majority being osteoderms or isolated molariforms.[2][113] In 2023, an associated skeleton of G. cylindricum, including skull and limb elements, from Guatemala was described, the most complete specimen from the region.[114] It is most likely that the first Glyptotherium populations started in Central America during the Great American Biotic Interchange in the Late Pliocene based on their paleobiogeography.[2][21] Glyptotherium fossils from Central America are sometimes placed as an indeterminate species,[23][22] but most are placed in Glyptotherium cylindricum or its synonyms.[108][109] This referral is also based on the age of the fossils, as the age of G. texanum fossils are measured to range from the Pliocene to early Pleistocene while the age of G. cylindricum fossils are confined to the late Pleistocene.[2][115]
Although commonly regarded as an exclusively North American genus,[115][22][2] fossils of Glyptotherium from northern South America in areas like Brazil and Venezuela have been discovered.[22][26] The fossils from South America are usually only osteoderms or caudal rings and are sometimes indeterminate on a species level, but are most likely from Glyptotherium cylindricum.[2][22][26]
Extinction
The chronology of megafaunal extinctions (such as Glyptotherium) in the Late Pleistocene extinctions has been disputed.[70] In the United States, the last reliable direct radiocarbon date for Glyptotherium is 23,230 ± 490 BP, from Laubach Cave No. 3, Texas.[116][70] Glyptotherium groups together with Eremotherium, Holmesina, and Paramylodon as having reliable final dates before the end of the Last Glacial Maximum of North America.[117] However, statistical analyses suggest that a later survival until the terminal Pleistocene of the United States is possible, based on sampling biases associated with uncommon fauna, and a lack of reliable dates from the humid Atlantic plain due to poor preservation.[70]
In South America, burnt Glyptotherium remains have been imprecisely dated to between 16,375 ± 400 BP and 14,300 ± 500 radiocarbon BP at Muaco, Venezuela,[26] with similar techniques dating the a Glyptotherium specimen from Taima-Taima to 12,580 ± 60 radiocarbon BP,[118][119] although a minimum date of the entire assemblage (~15,780 cal. BP, 12,980 ± 85 radiocarbon BP) is more recent.[26] As with other extinct Pleistocene megafauna, potential causes of extinction include human hunting, and climate change associated with the Younger Dryas cold interval.[70]
See also
References
- ↑ 1.0 1.1 1.2 Cuatáparo, J. N., & Ramírez, S. (1875). Descripción de un mamífero fósil de especie desconocida perteneciente al género" Glyptodon": encontrado entre las capas post-terciarias de Tequisquiac, en el Distrito de Zumpango. F. Diaz de Leon.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 2.26 2.27 2.28 2.29 2.30 2.31 2.32 2.33 2.34 2.35 2.36 2.37 2.38 2.39 2.40 2.41 2.42 Zurita, Alfredo Eduardo; Gillette, David D.; Cuadrelli, Francisco; Carlini, Alfredo Armando (2018-06-01). "A tale of two clades: Comparative study of Glyptodon Owen and Glyptotherium Osborn (Xenarthra, Cingulata, Glyptodontidae)". Geobios 51 (3): 247–258. doi:10.1016/j.geobios.2018.04.004. ISSN 0016-6995. Bibcode: 2018Geobi..51..247Z. https://www.sciencedirect.com/science/article/pii/S0016699517301870.
- ↑ 3.0 3.1 3.2 3.3 3.4 Gillette & Ray 1981, p. 3.
- ↑ Gillette & Ray 1981, p. 16.
- ↑ Felix, J. P. (1899). Beiträge zur geologie und paläontologie der republik Mexico (Vol. 1). A. Felix.
- ↑ Maldonado, M. (1948). Los vertebrados fósiles del Cuaternario en México. Revista de la Sociedad Mexicana de Historia Natural, 9 (1-2): 1-35
- ↑ 7.0 7.1 7.2 7.3 7.4 Osborn, Henry Fairfield (1903) (in en). Glyptotherium Texanum: A New Glyptodont from the Lower Pleistocene of Texas. order of the Trustees, American Museum of Natural History. https://books.google.com/books?id=BMUrAAAAYAAJ&dq=glyptotherium+texanum&pg=PA489.
- ↑ Cope, E. D. (1889). the edentata of North America. The American Naturalist, 23(272), 657-664.
- ↑ Leidy, J. (1889). Description of vertebrate remains from Peace Creek, Florida. Transactions of the Wagner Free Institute of Science of Philadelphia, 2, 19-31.
- ↑ Gillette & Ray 1981, p. 6.
- ↑ Leidy, D. (1889). Fossil vertebrates from Florida. Proceedings of the Academy of Natural Sciences of Philadelphia, 96-97.
- ↑ 12.0 12.1 Gillette & Ray 1981, p. 14.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Brown, Barnum (1912). "Brachyostracon, a new genus of glyptodonts from Mexico." (in en-US). Bulletin of the AMNH 31 (17): 167–190. https://digitallibrary.amnh.org/handle/2246/1402.
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 Gillette, D. D.; Ray, C. E. (1981), Glyptodonts of North America, https://repository.si.edu/bitstream/handle/10088/1966/SCtP-0040-Lo_res.pdf?sequence=2&isAllowed=y
- ↑ Hay, O. P. (1923). The Pleistocene of North America and Its Vertebrated Animals from the States East of the Mississippi River and from the Canadian Provinces East of Longitude 95> O (No. 22). Carnegie institution of Washington.
- ↑ Gillette & Ray 1981, p. 6 & 14.
- ↑ 17.0 17.1 17.2 17.3 17.4 Simpson, G. G., & Holmes, W. W. (1929). Pleistocene mammalian fauna of the Seminole Field, Pinellas County, Florida. Bulletin of the AMNH; v. 56, article 8.
- ↑ 18.0 18.1 18.2 18.3 Meade, G. E. (1953). An early Pleistocene vertebrate fauna from Frederick, Oklahoma. The Journal of Geology, 61(5), 452-460.
- ↑ Gould, C. N. (1928). The fossil Glyptodon in the Frederick gravel beds. In Proceedings of the Oklahoma Academy of Science (pp. 148-150).
- ↑ Spier, Leslie (1928-02-10). "Concerning Man's Antiquity at Frederick, Oklahoma" (in en). Science 67 (1728): 160–161. doi:10.1126/science.67.1728.160. ISSN 0036-8075. PMID 17752885. Bibcode: 1928Sci....67..160S. https://www.science.org/doi/10.1126/science.67.1728.160.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 21.12 Gillette, David D.; Carranza-Castañeda, Óscar; White, Richard S.; Morgan, Gary S.; Thrasher, Larry C.; McCord, Robert; McCullough, Gavin (2016-06-01). "Ontogeny and Sexual Dimorphism of Glyptotherium texanum (Xenarthra, Cingulata) from the Pliocene and Pleistocene (Blancan and Irvingtonian NALMA) of Arizona, New Mexico, and Mexico" (in en). Journal of Mammalian Evolution 23 (2): 133–154. doi:10.1007/s10914-015-9309-6. ISSN 1573-7055. https://doi.org/10.1007/s10914-015-9309-6.
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 Oliveira, É. V., Porpino, K. D., & Baretto, A. (2010). On the presence of Glyptotherium in the Late Pleistocene of Northeastern Brazil, and the status of "Glyptodon" and "Chlamydotherium". Paleobiogeographic implications. Neues Jahrbuch fur Geologie und Palaontologie-Abhandlungen, 258(3), 353.
- ↑ 23.0 23.1 23.2 23.3 23.4 Lessa, Carlos Micael Bonfim; Gomes, Verônica Santos; Cherkinsky, Alexander; Dantas, Mário André Trindade (2021). "Isotopic paleoecology (δ13C, δ18O) of two megamammals assemblages from the late pleistocene of Brazilian intertropical region" (in en). Journal of South American Earth Sciences 112: 103576. doi:10.1016/j.jsames.2021.103576. ISSN 0895-9811. Bibcode: 2021JSAES.11203576L. https://www.sciencedirect.com/science/article/pii/S0895981121004223.
- ↑ 24.0 24.1 24.2 Porpino, Kleberson de O.; Fernicola, Juan C.; Bergqvist, Lílian P. (2010-05-18). "Revisiting the intertropical Brazilian species Hoplophorus euphractus (Cingulata, Glyptodontoidea) and the phylogenetic affinities of Hoplophorus". Journal of Vertebrate Paleontology 30 (3): 911–927. doi:10.1080/02724631003765735. ISSN 0272-4634. Bibcode: 2010JVPal..30..911P. https://doi.org/10.1080/02724631003765735.
- ↑ Lund, P. W. (1845): Conspectum-dasypodum. – Det Kongelige Dans- ke Videnskabernes Selskbas Naturvidenskabelige og Mathematiske Afhandlinger, 11: lxxxii-lxxxvi.
- ↑ 26.00 26.01 26.02 26.03 26.04 26.05 26.06 26.07 26.08 26.09 26.10 26.11 26.12 Carlini, Alfredo A.; Carrillo-Briceño, Jorge D.; Jaimes, Arturo; Aguilera, Orangel; Zurita, Alfredo E.; Iriarte, José; Sánchez-Villagra, Marcelo R. (2022-06-16). "Damaged glyptodontid skulls from Late Pleistocene sites of northwestern Venezuela: evidence of hunting by humans?". Swiss Journal of Palaeontology 141 (1): 11. doi:10.1186/s13358-022-00253-3. ISSN 1664-2384. Bibcode: 2022SwJP..141...11C.
- ↑ 27.0 27.1 27.2 27.3 Mitchell, K.J.; Scanferla, A.; Soibelzon, E.; Bonini, R.; Ochoa, J.; Cooper, A. (2016). "Ancient DNA from the extinct South American giant glyptodont Doedicurus sp. (Xenarthra: Glyptodontidae) reveals that glyptodonts evolved from Eocene armadillos". Molecular Ecology 25 (14): 3499–3508. doi:10.1111/mec.13695. PMID 27158910. Bibcode: 2016MolEc..25.3499M. http://sedici.unlp.edu.ar/handle/10915/101557.
- ↑ 28.0 28.1 Delsuc, F.; Gibb, G.C.; Kuch, M.; Billet, G.; Hautier, L.; Southon, J.; Rouillard, J.-M.; Fernicola, J.C. et al. (2016). "The phylogenetic affinities of the extinct glyptodonts". Current Biology 26 (4): R155–R156. doi:10.1016/j.cub.2016.01.039. PMID 26906483.
- ↑ Delsuc, F.; Gibb, G.C.; Kuch, M.; Billet, G.; Hautier, L.; Southon, J.; Rouillard, J.-M.; Fernicola, J. C. et al. (2016-02-22). "The phylogenetic affinities of the extinct glyptodonts". Current Biology 26 (4): R155–R156. doi:10.1016/j.cub.2016.01.039. PMID 26906483. https://hal.archives-ouvertes.fr/hal-01879335/file/Delsuc_Poinar_CB_Correspondence_Manuscript_with_SuppInfo_rev1.pdf.
- ↑ 30.0 30.1 Cuadrelli, Francisco; Zurita, Alfredo E.; Toriño, Pablo; Miño-Boilini, Ángel R.; Rodríguez-Bualó, Santiago; Perea, Daniel; Acuña Suárez, Gabriel E. (2018-09-03). "Late Pleistocene Glyptodontinae (Mammalia, Xenarthra, Glyptodontidae) from southern South America: a comprehensive review". Journal of Vertebrate Paleontology 38 (5): e1525390. doi:10.1080/02724634.2018.1525390. ISSN 0272-4634. Bibcode: 2018JVPal..38E5390C. https://doi.org/10.1080/02724634.2018.1525390.
- ↑ Zurita, Alfredo E.; González Ruiz, Laureano R.; Gómez-Cruz, Arley J.; Arenas-Mosquera, Jose E. (2013-05-01). "The most complete known Neogene Glyptodontidae (Mammalia, Xenarthra, Cingulata) from northern South America: taxonomic, paleobiogeographic, and phylogenetic implications". Journal of Vertebrate Paleontology 33 (3): 696–708. doi:10.1080/02724634.2013.726677. ISSN 0272-4634. Bibcode: 2013JVPal..33..696Z. https://doi.org/10.1080/02724634.2013.726677.
- ↑ 32.0 32.1 Zurita, Alfredo E.; Miño-Boilini, Ángel R.; Francia, Analía; Arenas-Mosquera, José E. (2012-12-31). "The Pleistocene Glyptodontidae Gray, 1869 (Xenarthra: Cingulata) of Colombia and some considerations about the South American Glyptodontinae". Revista Brasileira de Paleontologia 15 (3): 273–280. doi:10.4072/rbp.2012.3.04. http://www.sbpbrasil.org/revista/edicoes/15_3/04_Zurita_et_al.pdf.
- ↑ 33.0 33.1 33.2 Cuadrelli, Francisco; Zurita, Alfredo E.; Toriño, Pablo; Miño-Boilini, Ángel R.; Perea, Daniel; Luna, Carlos A.; Gillette, David D.; Medina, Omar (2020-09-16). "A new species of glyptodontine (Mammalia, Xenarthra, Glyptodontidae) from the Quaternary of the Eastern Cordillera, Bolivia: phylogeny and palaeobiogeography". Journal of Systematic Palaeontology 18 (18): 1543–1566. doi:10.1080/14772019.2020.1784300. ISSN 1477-2019. Bibcode: 2020JSPal..18.1543C. https://doi.org/10.1080/14772019.2020.1784300.
- ↑ Gillette & Ray 1981, p. 2.
- ↑ Gillette & Ray 1981, p. 1.
- ↑ 36.0 36.1 Dantas, Mário André Trindade; Cherkinsky, Alexander; Lessa, Carlos Micael Bonfim; Santos, Luciano Vilaboim; Cozzuol, Mario Alberto; Omena, Érica Cavalcante; Silva, Jorge Luiz Lopes da; Sial, Alcides Nóbrega; Bocherens, Hervé (2018-11-29). "Integrative isotopic Paleoecology (δ13C, δ18O) of a Late Pleistocene vertebrate community from Sergipe, NE Brazil". p. 482752. bioRxiv 10.1101/482752.S2CID:91321429
- ↑ 37.0 37.1 37.2 du Plessis, Anton; Broeckhoven, Chris; Yadroitsev, Igor; Yadroitsava, Ina; le Roux, Stephan Gerhard (2018-06-01). "Analyzing nature's protective design: The glyptodont body armor" (in en). Journal of the Mechanical Behavior of Biomedical Materials 82: 218–223. doi:10.1016/j.jmbbm.2018.03.037. ISSN 1751-6161. PMID 29621689. https://www.sciencedirect.com/science/article/pii/S1751616118301656.
- ↑ Gillette & Ray 1981, p. 200.
- ↑ Gillette & Ray 1981, p. 58.
- ↑ Gillette & Ray 1981, p. 39.
- ↑ 41.0 41.1 41.2 Gillette & Ray 1981, p. 202.
- ↑ Zurita, A. E., Miño-Boilini, Á. R., Soibelzon, E., Carlini, A. A., & Paredes Rios, F. (2009). The diversity of Glyptodontidae (Xenarthra, Cingulata) in the Tarjia Valley (Bolivia): Systematic, biostratigraphic and paleobiogeographic aspects of a particular assemblage.(With 3 figures and 1 table). Neues Jahrbuch fur Geologie und Palaontologie-Abhandlungen, 251(2), 225.
- ↑ Zurita, A.E.; Scarano, A.C.; Carlini, A.A.; Scillato-Yané, G.J.; Soibelzon, E. (2011-04-04). "Neosclerocalyptus spp. (Cingulata: Glyptodontidae: Hoplophorini): cranial morphology and palaeoenvironments along the changing Quaternary". Journal of Natural History 45 (15–16): 893–914. doi:10.1080/00222933.2010.536917. ISSN 0022-2933. Bibcode: 2011JNatH..45..893Z. https://doi.org/10.1080/00222933.2010.536917.
- ↑ Fernicola, Juan Carlos; Toledo, Néstor; Bargo, M. Susana; Vizcaíno, Sergio F. (2012-09-22). "A neomorphic ossification of the nasal cartilages and the structure of paranasal sinus system of the glyptodont Neosclerocalyptus Paula Couto 1957 (Mammalia, Xenarthra)" (in English). Palaeontologia Electronica 15 (3): 1–22. doi:10.26879/333. ISSN 1094-8074. https://palaeo-electronica.org/content/2012-issue-3-articles/305-glyptodont-nasal-anatomy.
- ↑ 45.0 45.1 Zurita, Alfredo E.; González Ruiz, Laureano R.; Gómez-Cruz, Arley J.; Arenas-Mosquera, Jose E. (2013-05-01). "The most complete known Neogene Glyptodontidae (Mammalia, Xenarthra, Cingulata) from northern South America: taxonomic, paleobiogeographic, and phylogenetic implications". Journal of Vertebrate Paleontology 33 (3): 696–708. doi:10.1080/02724634.2013.726677. ISSN 0272-4634. Bibcode: 2013JVPal..33..696Z. https://doi.org/10.1080/02724634.2013.726677.
- ↑ 46.0 46.1 46.2 Zurita, Alfredo Eduardo; Soibelzon, Leopoldo Hector; Soibelzon, Esteban; Gasparini, Germán Mariano; Cenizo, Marcos Martín; Arzani, Héctor (2010-01-01). "Accessory protection structures in Glyptodon Owen (Xenarthra, Cingulata, Glyptodontidae)" (in en). Annales de Paléontologie 96 (1): 1–11. doi:10.1016/j.annpal.2010.01.001. ISSN 0753-3969. Bibcode: 2010AnPal..96....1Z. https://www.sciencedirect.com/science/article/pii/S0753396910000029.
- ↑ Gillette & Ray 1981, p. 169, 172, & 173.
- ↑ 48.0 48.1 48.2 48.3 48.4 Amson, Eli; Nyakatura, John A. (2018-12-01). "The Postcranial Musculoskeletal System of Xenarthrans: Insights from over Two Centuries of Research and Future Directions" (in en). Journal of Mammalian Evolution 25 (4): 459–484. doi:10.1007/s10914-017-9408-7. ISSN 1573-7055.
- ↑ 49.0 49.1 49.2 Owen, Richard (1841). "VI.— Description of a Tooth and Part of the Skeleton of the Glyptodon clavipes , a large Quadruped of the Edentate Order, to which belongs the Tesselated Bony Armour described and figured by Mr. Clift in the former Volume of the Transactions of the Geological Society; with a consideration of the question whether the Megatherium possessed an analogous Dermal Armour." (in en). Transactions of the Geological Society of London 6 (1): 81–106. doi:10.1144/transgslb.6.1.81. ISSN 2042-5295. https://www.lyellcollection.org/doi/10.1144/transgslb.6.1.81.
- ↑ Sénéchal, D. L. (1865). Notice sur l'armure ou le dermato-squelette et le système dentaire du Glyptodon clavipes, et particularités biologiques de cet animal, déduites d'après l'étude de ses restes fossiles. Balitout, Questroy et Cie.
- ↑ Fari a, R. A., & Vizcaíno, S. F. (1997). Allometry of the bones of living and extinct armadillos (Xenarthra, Dasypoda). Zeitschrift fur Saugetierkunde, 62, 65-70.
- ↑ Fariña, Richard A.; Vizcaı́no, Sergio F.; Blanco, R. Ernesto (1997-04-21). "Scaling of the Indicator of Athletic Capability in Fossil and Extant Land Tetrapods" (in en). Journal of Theoretical Biology 185 (4): 441–446. doi:10.1006/jtbi.1996.0323. ISSN 0022-5193. Bibcode: 1997JThBi.185..441F. https://www.sciencedirect.com/science/article/pii/S0022519396903239.
- ↑ Shockey, B. J. (2001). Specialized knee joints in some extinct, endemic, South American herbivores. Acta Palaeontologica Polonica, 46(2).
- ↑ 54.0 54.1 Russell, Dale A.; Rich, Fredrick J.; Schneider, Vincent; Lynch-Stieglitz, Jean (May 2009). "A warm thermal enclave in the Late Pleistocene of the South-eastern United States" (in en). Biological Reviews 84 (2): 173–202. doi:10.1111/j.1469-185X.2008.00069.x. ISSN 1464-7931. PMID 19391200. https://onlinelibrary.wiley.com/doi/10.1111/j.1469-185X.2008.00069.x.
- ↑ 55.0 55.1 55.2 55.3 55.4 55.5 Pérez-Crespo, V. A., Arroyo-Cabrales, J., Alva-Valdivia, L. M., Morales-Puente, P., & Cienfuegos-Alvarado, E. (2012). Diet and habitat definitions for Mexican glyptodonts from Cedral (San Luis Potosí, México) based on stable isotope analysis. Geological Magazine, 149(1), 153-157.
- ↑ 56.0 56.1 Keeley, J. E., & Rundel, P. W. (2003). Evolution of CAM and C4 carbon-concentrating mechanisms. International journal of plant sciences, 164(S3), S55-S77.
- ↑ 57.0 57.1 Omena, Érica Cavalcante; Silva, Jorge Luiz Lopes da; Sial, Alcides Nóbrega; Cherkinsky, Alexander; Dantas, Mário André Trindade (2021-10-03). "Late Pleistocene meso-megaherbivores from Brazilian Intertropical Region: isotopic diet (δ13C), niche differentiation, guilds and paleoenvironmental reconstruction (δ13C, δ18O)". Historical Biology 33 (10): 2299–2304. doi:10.1080/08912963.2020.1789977. ISSN 0891-2963. Bibcode: 2021HBio...33.2299O. https://doi.org/10.1080/08912963.2020.1789977.
- ↑ Dantas, M. A. T., Cherkinsky, A., Lessa, C. M. B., Santos, L. V., Cozzuol, M. A., Omena, É. C., ... & Bocherens, H. (2020). Isotopic paleoecology (δ13C, δ18O) of a late Pleistocene vertebrate community from the Brazilian Intertropical Region. Revista Brasileira de Paleontologia, 23(2), 138-152.
- ↑ Phillip E. Jardine, Christine M. Janis, Sarda Sahney, Michael J. Benton. "Grit not grass: Concordant patterns of early origin of hypsodonty in Great Plains ungulates and Glires." Palaeogeography, Palaeoclimatology, Palaeoecology. December 2012:365–366, 1–10
- ↑ De Iuliis, Gerardo; Bargo, María S.; Vizcaíno, Sergio F. (2001-01-19). [0743:VISMAM2.0.CO;2 "Variation in skull morphology and mastication in the fossil giant armadillos Pampatherium spp. and allied genera (Mammalia: Xenarthra: Pampatheriidae), with comments on their systematics and distribution"]. Journal of Vertebrate Paleontology 20 (4): 743–754. doi:10.1671/0272-4634(2000)020[0743:VISMAM2.0.CO;2]. ISSN 0272-4634. https://doi.org/10.1671/0272-4634(2000)020[0743:VISMAM]2.0.CO;2.
- ↑ Vizcaíno, Sergio F.; Cassini, Guillermo H.; Fernicola, Juan C.; Bargo, M. Susana (2011). "Evaluating Habitats and Feeding Habits Through Ecomorphological Features in Glyptodonts (Mammalia, Xenarthra)". Ameghiniana: 305–319. doi:10.5710/AMGH.v48i3(364). S2CID:85793531. Retrieved 2015-10-29.
- ↑ Vizcaíno, S. F., Fariña, R. A., & Mazzetta, G. V. (1999). Ulnar dimensions and fossoriality in armadillos. Acta Theriologica, 44.
- ↑ Carter, T. S., & Encarnaçao, C. D. (1983). Characteristics and use of burrows by four species of armadillos in Brazil. Journal of Mammalogy, 64(1), 103-108.
- ↑ Vizcaíno, Sergio F.; Fariña, Richard A. (2007-03-29). "Diet and locomotion of the armadillo Peltephilus: a new view" (in en). Lethaia 30 (1): 79–86. doi:10.1111/j.1502-3931.1997.tb00446.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1502-3931.1997.tb00446.x.
- ↑ Saarinen, Juha; Karme, Aleksis (2017-06-15). "Tooth wear and diets of extant and fossil xenarthrans (Mammalia, Xenarthra) – Applying a new mesowear approach" (in en). Palaeogeography, Palaeoclimatology, Palaeoecology 476: 42–54. doi:10.1016/j.palaeo.2017.03.027. ISSN 0031-0182. Bibcode: 2017PPP...476...42S. https://www.sciencedirect.com/science/article/pii/S0031018216306630.
- ↑ Vizcaíno, Sergio F.; Blanco, R. Ernesto; Bender, J. Benjamín; Milne, Nick (2011). "Proportions and function of the limbs of glyptodonts: Glyptodont limbs" (in en). Lethaia 44 (1): 93–101. doi:10.1111/j.1502-3931.2010.00228.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1502-3931.2010.00228.x.
- ↑ Chen, Irene H.; Kiang, James H.; Correa, Victor; Lopez, Maria I.; Chen, Po-Yu; McKittrick, Joanna; Meyers, Marc A. (2011-07-01). "Armadillo armor: Mechanical testing and micro-structural evaluation" (in en). Journal of the Mechanical Behavior of Biomedical Materials. Special Issue on Natural Materials / Papers from the Third International Conference on the Mechanics of Biomaterials and Tissues 4 (5): 713–722. doi:10.1016/j.jmbbm.2010.12.013. ISSN 1751-6161. PMID 21565719. https://www.sciencedirect.com/science/article/pii/S1751616110001888.
- ↑ Barbosa, Fernando Henrique de Souza; Porpino, Kleberson de Oliveira; Fragoso, Ana Bernadete Lima; Oliveira, Edison Vicente (2014-02-13). "Arthritis in a Glyptodont (Mammalia, Xenarthra, Cingulata)" (in en). PLOS ONE 9 (2): e88646. doi:10.1371/journal.pone.0088646. ISSN 1932-6203. PMID 24551126. Bibcode: 2014PLoSO...988646B.
- ↑ Lima, Fábio Cunha Guimarães de; Porpino, Kleberson de Oliveira (2018-10-18). "Ectoparasitism and infections in the exoskeletons of large fossil cingulates" (in en). PLOS ONE 13 (10): e0205656. doi:10.1371/journal.pone.0205656. ISSN 1932-6203. PMID 30335796. Bibcode: 2018PLoSO..1305656D.
- ↑ 70.0 70.1 70.2 70.3 70.4 Faith, J. Tyler; Surovell, Todd A. (2009-12-08). "Synchronous extinction of North America's Pleistocene mammals" (in en). Proceedings of the National Academy of Sciences 106 (49): 20641–20645. doi:10.1073/pnas.0908153106. ISSN 0027-8424. PMID 19934040. Bibcode: 2009PNAS..10620641F.
- ↑ Repenning, Charles A. (1962). "The Giant Ground Squirrel Paenemarmota". Journal of Paleontology. 36 (93): 540–556. JSTOR 1301086.
- ↑ 72.0 72.1 Dalquest, W. W. (1975). Vertebrate fossils from the Blanco local fauna of Texas.
- ↑ Martin, L. D., Schultz, C. B., & Schultz, M. R. (1988). Saber-toothed cats from the Plio-Pleistocene of Nebraska.
- ↑ Feduccia, J. Alan; Ford, Norman L. (1970-10-01). "Some birds of prey from the Upper Pliocene of Kansas". The Auk 87 (4): 795–797. doi:10.2307/4083714. ISSN 1938-4254. https://doi.org/10.2307/4083714.
- ↑ Marshall, William H. (1937-01-01). "Double-crested Cormorant Nesting on the Bear River Refuge in Utah". The Condor 39 (1): 36. doi:10.2307/1363487. ISSN 1938-5129. https://doi.org/10.2307/1363487.
- ↑ Dávila, S. Lorena; Stinnesbeck, Sarah R.; Gonzalez, Silvia; Lindauer, Susanne; Escamilla, Juan; Stinnesbeck, Wolfgang (2019-09-01). "Guatemala's Late Pleistocene (Rancholabrean) fauna: Revision and interpretation" (in en). Quaternary Science Reviews 219: 277–296. doi:10.1016/j.quascirev.2019.07.011. ISSN 0277-3791. Bibcode: 2019QSRv..219..277D. https://www.sciencedirect.com/science/article/pii/S0277379119302318.
- ↑ Stinnesbeck, S. R. (2020). Mexican fossil ground sloths-A case study for Late Pleistocene megafaunal turnover in the Mexican Corridor.
- ↑ 78.0 78.1 78.2 Cartelle, Castor; Hartwig, W. C. (1996). "A new extinct primate among the Pleistocene megafauna of Bahia, Brazil". Proceedings of the National Academy of Sciences. 93 (13): 6405–6409.
- ↑ Eisenberg, John F.; Redford, Kent H. (1989). Mammals of the Neotropics, Volume 3: Ecuador, Bolivia, Brazil. University of Chicago Press. p. 247. Script error: No such module "CS1 identifiers"..
- ↑ Halenar, Lauren B. (December 2011). "Reconstructing the Locomotor Repertoire of Protopithecus brasiliensis". The Anatomical Record. 294 (12): 2048–2063.
- ↑ 81.0 81.1 David Webb, S. (2006-08-23). "The Great American Biotic Interchange: Patterns and Processes1" (in en). Annals of the Missouri Botanical Garden 93 (2): 245–257. doi:10.3417/0026-6493(2006)93[245:TGABIP2.0.CO;2]. ISSN 0026-6493. http://www.bioone.org/doi/abs/10.3417/0026-6493%282006%2993%5B245%3ATGABIP%5D2.0.CO%3B2.
- ↑ Charrier, John J. Flynn, André R. Wyss and Reynaldo (2007). "South America's Missing Mammals" (in en). Scientific American 296 (5): 68–75. doi:10.1038/scientificamerican0507-68. PMID 17500416. Bibcode: 2007SciAm.296e..68F. https://www.scientificamerican.com/article/south-americas-missing-mammals/. Retrieved 2023-05-09.
- ↑ Bloch, J. I., Woodruff, E. D., Wood, A. R., Rincon, A. F., Harrington, A. R., Morgan, G. S., ... & MacFadden, B. J. (2016). First North American fossil monkey and early Miocene tropical biotic interchange. Nature, 533(7602), 243-246.
- ↑ Croft, D. A. (2016). Horned armadillos and rafting monkeys: the fascinating fossil mammals of South America. Indiana University Press.
- ↑ Morgan, G. S. (2005). The great American biotic interchange in Florida. Bulletin of the Florida Museum of Natural History, 45(4), 271-311.
- ↑ McDonald, H. G. (2005). Palecology of extinct xenarthrans and the Great American Biotic Interchange. Bulletin of the Florida Museum of Natural History, 45(4), 319-340.
- ↑ 87.0 87.1 Scillato-Yané, G. J.; Carlini, A. A.; Tonni, E. P.; Noriega, J. I. (2005-10-01). "Paleobiogeography of the late Pleistocene pampatheres of South America" (in en). Journal of South American Earth Sciences. Quaternary Paleontology and biostratigraphy of southern South Africa 20 (1): 131–138. doi:10.1016/j.jsames.2005.06.012. ISSN 0895-9811. Bibcode: 2005JSAES..20..131S. https://www.sciencedirect.com/science/article/pii/S0895981105001288.
- ↑ 88.0 88.1 88.2 Carlini, Alfredo A.; Zurita, Alfredo E.; Aguilera, Orangel A. (2008). "North American Glyptodontines (Xenarthra, Mammalia) in the Upper Pleistocene of northern South America" (in en). Paläontologische Zeitschrift 82 (2): 125–138. doi:10.1007/BF02988404. ISSN 0031-0220. Bibcode: 2008PalZ...82..125C. https://doi.org/10.1007/BF02988404.
- ↑ 89.0 89.1 Cione, A. L., Gasparini, G. M., Soibelzon, E., Soibelzon, L. H., & Tonni, E. P. (2015). The great American biotic interchange: a South American perspective (p. 97). Dordrecht: Springer.
- ↑ Woodburne, M. O. (2010). The Great American Biotic Interchange: dispersals, tectonics, climate, sea level and holding pens. Journal of mammalian evolution, 17, 245-264.
- ↑ Lundelius, Ernest L.; Bryant, Vaughn M.; Mandel, Rolfe; Thies, Kenneth J.; Thoms, Alston (2013-01-01). "The first occurrence of a toxodont (Mammalia, Notoungulata) in the United States". Journal of Vertebrate Paleontology 33 (1): 229–232. doi:10.1080/02724634.2012.711405. ISSN 0272-4634. Bibcode: 2013JVPal..33..229L. https://doi.org/10.1080/02724634.2012.711405.
- ↑ 92.0 92.1 Zurita, Alfredo E.; Carlini, Alfredo A.; Gillette, David; Sánchez, Rodolfo (2011-03-01). "Late Pliocene Glyptodontinae (Xenarthra, Cingulata, Glyptodontidae) of South and North America: Morphology and paleobiogeographical implications in the GABI". Journal of South American Earth Sciences 31 (2): 178–185. doi:10.1016/j.jsames.2011.02.001. ISSN 0895-9811. Bibcode: 2011JSAES..31..178Z. https://www.sciencedirect.com/science/article/pii/S0895981111000095.
- ↑ Pujos, F., & Salas, R. (2004). A systematic reassessment and paleogeographic review of fossil Xenarthra from Peru. Bulletin de l'Institut français d'études andines, (33 (2)), 331-377.
- ↑ Rabassa, Jorge; Coronato, Andrea M.; Salemme, Mónica (2005-10-01). "Chronology of the Late Cenozoic Patagonian glaciations and their correlation with biostratigraphic units of the Pampean region (Argentina)" (in en). Journal of South American Earth Sciences. Quaternary Paleontology and biostratigraphy of southern South Africa 20 (1): 81–103. doi:10.1016/j.jsames.2005.07.004. ISSN 0895-9811. Bibcode: 2005JSAES..20...81R. https://www.sciencedirect.com/science/article/pii/S0895981105001276.
- ↑ Antón, Mauricio (2013). Sabertooth. Bloomington, Indiana: University of Indiana Press. pp. 203–204. ISBN 978-0-253-01042-1.
- ↑ Gillette, D. D. (Spring 2010). "Glyptodonts in Arizona". Arizona Geology (Arizona Geological Survey). http://azgeology.azgs.arizona.edu/archived_issues/azgs.az.gov/arizona_geology/spring10/article_feature.html. Retrieved 2018-08-17.
- ↑ 97.0 97.1 Brandes, G. (1900) : Ueber eine Ursache des Aussterbens Diluvialer Säugethiere. Corrblatt d. Deutsch . Ges. f. Anthropol. Jahrg. 31. Munichen 1901.
- ↑ 98.0 98.1 Bohlin, B. (1940). 8. Food habit of the machairodonts, with special regard to Smilodon.
- ↑ Araújo-Júnior, Hermínio Ismael de; Barbosa, Fernando Henrique de Souza; Silva, Lucas Henrique Medeiros da (2017-02-15). "Overlapping paleoichnology, paleoecology and taphonomy: Analysis of tooth traces in a Late Pleistocene-early Holocene megafaunal assemblage of Brazil and description of a new ichnotaxon in hard substrate" (in en). Palaeogeography, Palaeoclimatology, Palaeoecology 468: 122–128. doi:10.1016/j.palaeo.2016.12.007. ISSN 0031-0182. Bibcode: 2017PPP...468..122A. https://www.sciencedirect.com/science/article/pii/S0031018216308379.
- ↑ Crook, Wilson W.; Harris, R. K. (1958). "A Pleistocene Campsite near Lewisville, Texas" (in en). American Antiquity 23 (3): 233–246. doi:10.2307/276304. ISSN 0002-7316. https://www.cambridge.org/core/journals/american-antiquity/article/abs/pleistocene-campsite-near-lewisville-texas/D4C1AFD4056ABC2C66B5599CFCE8938C.
- ↑ Waguespack, Nicole M.; Surovell, Todd A. (2003). "Clovis Hunting Strategies, or How to Make out on Plentiful Resources" (in en). American Antiquity 68 (2): 333–352. doi:10.2307/3557083. ISSN 0002-7316. https://www.cambridge.org/core/journals/american-antiquity/article/abs/clovis-hunting-strategies-or-how-to-make-out-on-plentiful-resources/F5D7B9E8721919E3E3823C9BF89E37FA.
- ↑ Vogt, C. (1881). Squelette humain associe aux glyptodontidae. Bulletin de la Societe d'Antropologie de Paris, 3(4), 693–699
- ↑ Quiñones, Sofía I.; De los Reyes, Martin; Zurita, Alfredo E.; Cuadrelli, Francisco; Miño-Boilini, Ángel R.; Poiré, Daniel G. (2020-11-01). "Neosclerocalyptus Paula Couto (Xenarthra, Glyptodontidae) in the late Pliocene-earliest Pleistocene of the Pampean region (Argentina): Its contribution to the understanding of evolutionary history of Pleistocene glyptodonts" (in en). Journal of South American Earth Sciences 103: 102701. doi:10.1016/j.jsames.2020.102701. ISSN 0895-9811. Bibcode: 2020JSAES.10302701Q. https://www.sciencedirect.com/science/article/pii/S0895981120302443.
- ↑ Politis, Gustavo G.; Messineo, Pablo G.; Stafford, Thomas W.; Lindsey, Emily L. (2019). "Campo Laborde: A Late Pleistocene giant ground sloth kill and butchering site in the Pampas" (in en). Science Advances 5 (3): eaau4546. doi:10.1126/sciadv.aau4546. ISSN 2375-2548. PMID 30854426. Bibcode: 2019SciA....5.4546P.
- ↑ Prates, Luciano; Perez, S. Ivan (2021-04-12). "Late Pleistocene South American megafaunal extinctions associated with rise of Fishtail points and human population" (in en). Nature Communications 12 (1): 2175. doi:10.1038/s41467-021-22506-4. ISSN 2041-1723. PMID 33846353. Bibcode: 2021NatCo..12.2175P.
- ↑ 106.0 106.1 106.2 Albright, L.; Sanders, Albert; Weems, Robert; Cicimurri, David; Knight, James (2019-10-31). "Cenozoic vertebrate biostratigraphy of South Carolina, U.S.A., and additions to the fauna" (in en). Bulletin of the Florida Museum of Natural History 57 (2): 77–236. doi:10.58782/flmnh.qqgg4577. ISSN 2373-9991. https://flmnhbulletin.com/index.php/flmnh/article/view/flmnh-vol57-no2.
- ↑ Sanders, A. E. (2002). Additions to the Pleistocene mammal faunas of South Carolina, North Carolina, and Georgia. American Philosophical Society.
- ↑ 108.0 108.1 Dávila, S. Lorena; Stinnesbeck, Sarah R.; Gonzalez, Silvia; Lindauer, Susanne; Escamilla, Juan; Stinnesbeck, Wolfgang (2019-09-01). "Guatemala's Late Pleistocene (Rancholabrean) fauna: Revision and interpretation" (in en). Quaternary Science Reviews 219: 277–296. doi:10.1016/j.quascirev.2019.07.011. ISSN 0277-3791. Bibcode: 2019QSRv..219..277D. https://www.sciencedirect.com/science/article/pii/S0277379119302318.
- ↑ 109.0 109.1 Valerio, A. L., & Laurito, C. A. (2011). El registro fósil de Glyptotherium floridanum (Xenarthra, Cingulata, Glyptodontidae) en el Cuaternario de Costa Rica, América Central. Revista Geológica de América Central, (45), 141-145.
- ↑ Jackson, D. R., & Fernandez, E. (2005). A small Pleistocene mammalian megafauna from southern Honduras. Bulletin of the Florida Museum of Natural History, 45(4), 261-269.
- ↑ Cisneros, J. C. (2005). New pleistocene vertebrate fauna from El Salvador. Revista Brasileira de Paleontologia, 8(3), 239-255.
- ↑ Lucas, Spencer G. (2014). "Late pleistocene mammals from El Hatillo, Panama" (in en). Revista Geológica de América Central (50): 139–151. ISSN 0256-7024. http://www.scielo.sa.cr/scielo.php?script=sci_abstract&pid=S0256-70242014000100007&lng=en&nrm=iso&tlng=en.
- ↑ Valerio, Ana L.; Laurito, César A. (2011). "El registro fósil de Glyptotherium Floridanum (Xenarthra, Cingulata, Glyptodontidae) en el Cuaternario de Costa Rica, América Central" (in es). Revista Geológica de América Central (45): 141–145. ISSN 0256-7024. http://www.scielo.sa.cr/scielo.php?script=sci_abstract&pid=S0256-70242011000200008&lng=en&nrm=iso&tlng=es.
- ↑ Cuadrelli, Francisco; Escamilla, Juan; Zurita, Alfredo; Gillette, David D.; Dávila, Lorena S. (2023-08-22). "Glyptotherium cylindricum (Cingulata, Glyptodontidae) from the Late Pleistocene of Guatemala: the most complete record of Glyptodontinae from Central America" (in en). Alcheringa: An Australasian Journal of Palaeontology 47 (3): 336–347. doi:10.1080/03115518.2023.2242440. ISSN 0311-5518. Bibcode: 2023Alch...47..336C. https://www.tandfonline.com/doi/full/10.1080/03115518.2023.2242440.
- ↑ 115.0 115.1 Ramírez-Cruz, Gonzalo A.; Montellano-Ballesteros, Marisol (2014). "Two new glyptodont records (Mammalia: Cingulata) from the late Pleistocene of Tamaulipas and Tlaxcala, Mexico: Implications for the taxonomy of the genus Glyptotherium". The Southwestern Naturalist 59 (4): 522–530. doi:10.1894/JKF-45.1. ISSN 0038-4909. https://bioone.org/journals/the-southwestern-naturalist/volume-59/issue-4/JKF-45.1/Two-new-glyptodont-records-Mammalia--Cingulata-from-the-late/10.1894/JKF-45.1.full.
- ↑ Haynes, Gary (2013-02-08). "Extinctions in North America's Late Glacial landscapes". Quaternary International. Peopling the last new worlds: the first colonisation of Sahul and the Americas 285: 89–98. doi:10.1016/j.quaint.2010.07.026. ISSN 1040-6182. Bibcode: 2013QuInt.285...89H. https://www.sciencedirect.com/science/article/pii/S104061821000296X.
- ↑ Stuart, Anthony John (May 2015). "Late Quaternary megafaunal extinctions on the continents: a short review" (in en). Geological Journal 50 (3): 338–363. doi:10.1002/gj.2633. ISSN 0072-1050. Bibcode: 2015GeolJ..50..338S. https://onlinelibrary.wiley.com/doi/10.1002/gj.2633.
- ↑ Carlini, A.A.; Zurita, A.E. (2006). "Glyptotherium Osborn (Mammalia, Xenarthra, Cingulata) en el Pleistoceno Tardío de Venezuela". 9° Congreso Argentino de Paleontología y Bioestratigrafía, Córdoba: 98.
- ↑ Faria, Fabio Henrique Cortes; Kinoshita, Angela; Carvalho, Ismar de Souza; Araújo-Júnior, Hermínio Ismael de; Pegorin, Priscila; Maria G Figueiredo, Ana; Baffa, Oswaldo (2020-08-01). "ESR dating of late Quaternary megafauna fossils from João Dourado, Bahia, Brazil". Journal of South American Earth Sciences 101: 102586. doi:10.1016/j.jsames.2020.102586. ISSN 0895-9811. https://www.sciencedirect.com/science/article/pii/S0895981120300997.
Wikidata ☰ Q138952 entry
 |
