Medicine:Periodontal disease
| Periodontal disease | |
|---|---|
| Other names | Gum disease, pyorrhea, periodontitis |
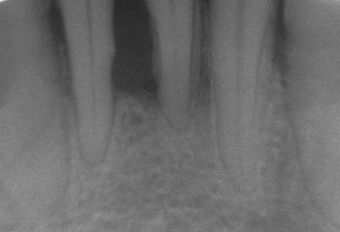 | |
| Radiograph showing bone loss between the two roots of a tooth (black region). The spongy bone has receded due to infection under tooth, reducing the bony support for the tooth. | |
| Pronunciation |
|
| Specialty | Dentistry |
| Symptoms | Red, swollen, painful, bleeding gums, loose teeth, bad breath[1] |
| Complications | Tooth loss, gum abscess[1][2] |
| Usual onset | Getting gingivitis[3] |
| Causes | Bacteria related plaque build up[1] |
| Risk factors | Smoking,[4] diabetes, HIV/AIDS, certain medications[1] |
| Diagnostic method | Dental examination, X-rays[1] |
| Treatment | Good oral hygiene, regular professional cleaning[5] |
| Frequency | 538 million (2015)[6] |
Periodontal disease, also known as gum disease, is a set of inflammatory conditions affecting the tissues surrounding the teeth.[5] In its early stage, called gingivitis, the gums become swollen and red and may bleed.[5] It is considered the main cause of tooth loss for adults worldwide.[7][8] In its more serious form, called periodontitis, the gums can pull away from the tooth, bone can be lost, and the teeth may loosen or fall out.[5] Bad breath may also occur.[1]
Periodontal disease is generally due to bacteria in the mouth infecting the tissue around the teeth.[5] Factors that increase the risk of disease include smoking,[4] diabetes, HIV/AIDS, family history, high levels of homocysteine in the blood and certain medications.[1] Diagnosis is by inspecting the gum tissue around the teeth both visually and with a probe and X-rays looking for bone loss around the teeth.[1][9]
Treatment involves good oral hygiene and regular professional teeth cleaning.[5] Recommended oral hygiene include daily brushing and flossing.[5] In certain cases antibiotics or dental surgery may be recommended.[10] Clinical investigations demonstrate that quitting smoking and making dietary changes enhance periodontal health.[11][12] Globally 538 million people were estimated to be affected in 2015 and has been known to affect 10–15% of the population generally.[7][8][6] In the United States nearly half of those over the age of 30 are affected to some degree, and about 70% of those over 65 have the condition.[5] Males are affected more often than females.[5] File:En.Wikipedia-VideoWiki-Periodontal disease.webm
Signs and symptoms
In the early stages, periodontitis has very few symptoms, and in many individuals the disease has progressed significantly before they seek treatment.
Symptoms may include:
- Redness or bleeding of gums while brushing teeth, using dental floss or biting into hard food (e.g., apples) (though this may also occur in gingivitis, where there is no attachment loss gum disease)
- Gum swelling that recurs
- Spitting out blood after brushing teeth
- Halitosis, or bad breath, and a persistent metallic taste in the mouth
- Gingival recession, resulting in apparent lengthening of teeth (this may also be caused by heavy-handed brushing or with a stiff toothbrush)
- Deep pockets between the teeth and the gums (pockets are sites where the attachment has been gradually destroyed by collagen-destroying enzymes, known as collagenases)
- Loose teeth, in the later stages (though this may occur for other reasons, as well)
Gingival inflammation and bone destruction are largely painless. Hence, people may wrongly assume painless bleeding after teeth cleaning is insignificant, although this may be a symptom of progressing periodontitis in that person.

Associated conditions
Periodontitis has been linked to increased inflammation in the body, such as indicated by raised levels of C-reactive protein and interleukin-6.[13][14][15][16] It is associated with an increased risk of stroke,[17][18] myocardial infarction,[19] atherosclerosis[20][21][22][23][24][25][26] and hypertension.[27] It also linked in those over 60 years of age to impairments in delayed memory and calculation abilities.[28][29] Individuals with impaired fasting glucose and diabetes mellitus have higher degrees of periodontal inflammation, and often have difficulties with balancing their blood glucose level owing to the constant systemic inflammatory state, caused by the periodontal inflammation.[30][31] Although no causal association was proven, there is an association between chronic periodontitis and erectile dysfunction,[32] inflammatory bowel disease,[33] heart disease,[34] and pancreatic cancer.[35]
Diabetes and periodontal disease
A positive correlation between raised levels of glucose within the blood and the onset or progression of periodontal disease has been shown in the current literature.
Data has also shown that there is a significant increase in the incidence or progression of periodontitis in patients with uncontrolled diabetes compared to those who do not have diabetes or have well-controlled diabetes. In uncontrolled diabetes, the formation of reactive oxygen species can damage cells such as those in the connective tissue of the periodontal ligament, resulting in cell necrosis or apoptosis. Furthermore, individuals with uncontrolled diabetes mellitus who have frequent exposure to periodontal pathogens have a greater immune response to these bacteria. This can subsequently cause and/or accelerate periodontal tissue destruction leading to periodontal disease.[36]
Oral cancer and periodontal disease
Current literature suggests a link between periodontal disease and oral cancer. Studies have confirmed an increase in systemic inflammation markers such as C-Reactive Protein and Interleukin-6 to be found in patients with advanced periodontal disease. The link between systemic inflammation and oral cancer has also been well established.
Both periodontal disease and cancer risk are associated with genetic susceptibility and it is possible that there is a positive association by a shared genetic susceptibility in the two diseases.
Due to the low incidence rate of oral cancer, studies have not been able to conduct quality studies to prove the association between the two, however future larger studies may aid in the identification of individuals at a higher risk.[37]
Systemic implications
Periodontal disease (PD) can be described as an inflammatory condition affecting the supporting structures of the teeth. Studies have shown that PD is associated with higher levels of systemic inflammatory markers such as Interleukin-6 (IL-6), C-Reactive Protein (CRP) and Tumor Necrosis Factor (TNF). To compare, elevated levels of these inflammatory markers are also associated with cardiovascular disease and cerebrovascular events such as ischemic strokes.[38]
The presence of a wide spectrum inflammatory oral diseases can increase the risk of an episode of stroke in an acute or chronic phase. Inflammatory markers, CRP, IL-6 are known risk factors of stroke. Both inflammatory markers are also biomarkers of PD and found to be an increased level after daily activities, such as mastication or toothbrushing, are performed. Bacteria from the periodontal pockets will enter the bloodstream during these activities and the current literature suggests that this may be a possible triggering of the aggravation of the stroke process.[39]
Other mechanisms have been suggested, PD is a known chronic infection. It can aid in the promotion of atherosclerosis by the deposition of cholesterol, cholesterol esters and calcium within the subendothelial layer of vessel walls.[40] Atherosclerotic plaque that is unstable may rupture and release debris and thrombi that may travel to different parts of the circulatory system causing embolization and therefore, an ischemic stroke. Therefore, PD has been suggested as an independent risk factor for stroke.
A variety of cardiovascular diseases can also be associated with periodontal disease. Patients with higher levels of inflammatory markers such as TNF, IL-1, IL-6 and IL-8 can lead to progression of atherosclerosis and the development and perpetuation of atrial fibrillation,[41] as it is associated with platelet and coagulation cascade activations, leading to thrombosis and thrombotic complications.
Experimental animal studies have shown a link between periodontal disease, oxidative stress and cardiac stress. Oxidative stress favours the development and progression of heart failure as it causes cellular dysfunction, oxidation of proteins and lipids, and damage to the deoxyribonucleic acid (DNA), stimulating fibroblast proliferation and metalloproteinases activation favouring cardiac remodelling.[42]
During SARS Covid 19 pandemic, Periodontitis was significantly associated with a higher risk of complications from COVID‐19, including ICU admission, need for assisted ventilation and death and increased blood levels of markers such as D‐dimer, WBC and CRP which are linked with worse disease outcome. [43]
Clinical significance
Inadequate nutrition and periodontal disease
Periodontal disease is multifactorial, and nutrition can significantly affect its prognosis. Studies have shown that a healthy and well-balanced diet is crucial to maintaining periodontal health. [11] Nutritional deficiencies can lead to oral manifestations such as those in scurvy and rickets disease. Different vitamins will play a different role in periodontal health:
- Vitamin C: Deficiencies may lead to gingival inflammation and bleeding, subsequently advancing periodontal disease
- Vitamin D: Deficiencies may lead to delayed post-surgical healing
- Vitamin E: Deficiencies may lead to impaired gingival wound healing
- Vitamin K: Deficiencies may lead to gingival bleeding
Nutritional supplements of vitamins have also been shown to positively affect healing after periodontal surgery and many of these vitamins can be found in a variety of food that we eat within a regular healthy diet. [11] Therefore, vitamin intakes (particularly vitamin C) and dietary supplements not only play a role in improving periodontal health, but also influence the rate of bone formation and periodontal regeneration. However, studies supporting the correlation between nutrition and periodontal health are limited, and more long-term research is required to confirm this. [44]
Causes
Periodontitis is an inflammation of the periodontium, i.e., the tissues that support the teeth. The periodontium consists of four tissues:
- gingiva, or gum tissue,
- cementum, or outer layer of the roots of teeth,
- alveolar bone, or the bony sockets into which the teeth are anchored, and
- periodontal ligaments (PDLs), which are the connective tissue fibers that run between the cementum and the alveolar bone.
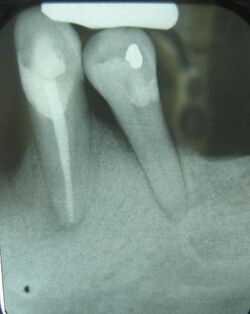
The primary cause of gingivitis is poor or ineffective oral hygiene,[45] which leads to the accumulation of a mycotic[46][47][48][49] and bacterial matrix at the gum line, called dental plaque. Other contributors are poor nutrition and underlying medical issues such as diabetes.[50] Diabetics must be meticulous with their homecare to control periodontal disease.[51] New finger prick tests have been approved by the Food and Drug Administration in the US, and are being used in dental offices to identify and screen people for possible contributory causes of gum disease, such as diabetes.
In some people, gingivitis progresses to periodontitis – with the destruction of the gingival fibers, the gum tissues separate from the tooth and deepened sulcus, called a periodontal pocket. Subgingival microorganisms (those that exist under the gum line) colonize the periodontal pockets and cause further inflammation in the gum tissues and progressive bone loss. Examples of secondary causes are those things that, by definition, cause microbic plaque accumulation, such as restoration overhangs and root proximity.
Smoking is another factor that increases the occurrence of periodontitis, directly or indirectly,[52][53][54] and may interfere with or adversely affect its treatment.[55][56][57] It is arguably the most important environmental risk factor for periodontitis. Research has shown that smokers have more bone loss, attachment loss and tooth loss compared to non-smokers.[58] This is likely due to several effects of smoking on the immune response including decreased wound healing, suppression of antibody production, and the reduction of phagocytosis by neutrophils[58]
Ehlers–Danlos syndrome and Papillon–Lefèvre syndrome (also known as palmoplantar keratoderma) are also risk factors for periodontitis.
If left undisturbed, microbial plaque calcifies to form calculus, which is commonly called tartar. Calculus above and below the gum line must be removed completely by the dental hygienist or dentist to treat gingivitis and periodontitis. Although the primary cause of both gingivitis and periodontitis is the microbial plaque that adheres to the tooth surfaces, there are many other modifying factors. A very strong risk factor is one's genetic susceptibility. Several conditions and diseases, including Down syndrome, diabetes, and other diseases that affect one's resistance to infection, also increase susceptibility to periodontitis.
Periodontitis may be associated with higher stress.[59] Periodontitis occurs more often in people from the lower end of the socioeconomic scale than people from the upper end of the socioeconomic scale.[60]
Genetics appear to play a role in determining the risk for periodontitis. It is believed genetics could explain why some people with good plaque control have advanced periodontitis, whilst some others with poor oral hygiene are free from the disease. Genetic factors which could modify the risk of a person developing periodontitis include:
- Defects of phagocytosis: person may have hypo-responsive phagocytes.
- Hyper-production of interleukins, prostaglandins and cytokines, resulting in an exaggerated immune response.
- Interleukin 1 (IL-1) gene polymorphism: people with this polymorphism produce more IL-1, and subsequently are more at risk of developing chronic periodontitis.[58]
Diabetes appears to exacerbate the onset, progression, and severity of periodontitis.[61] Although the majority of research has focused on type 2 diabetes, type 1 diabetes appears to have an identical effect on the risk for periodontitis.[62] The extent of the increased risk of periodontitis is dependent on the level of glycaemic control. Therefore, in well managed diabetes there seems to be a small effect of diabetes on the risk for periodontitis. However, the risk increases exponentially as glycaemic control worsens.[62] Overall, the increased risk of periodontitis in diabetics is estimated to be between two and three times higher.[61] So far, the mechanisms underlying the link are not fully understood, but it is known to involve aspects of inflammation, immune functioning, neutrophil activity, and cytokine biology.[62][63]
Mechanism
As dental plaque or biofilm accumulates on the teeth near and below the gums there is some dysbiosis of the normal oral microbiome.[64] As of 2017 it was not certain what species were most responsible for causing harm, but gram-negative anaerobic bacteria, spirochetes, and viruses have been suggested; in individual people it is sometimes clear that one or more species is driving the disease.[64] Research in 2004 indicated three gram negative anaerobic species: Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Bacteroides forsythus and Eikenella corrodens.[58]
Plaque may be soft and uncalcified, hard and calcified, or both; for plaques that are on teeth the calcium comes from saliva; for plaques below the gumline, it comes from blood via oozing of inflamed gums.[64]
The damage to teeth and gums comes from the immune system as it attempts to destroy the microbes that are disrupting the normal symbiosis between the oral tissues and the oral microbe community. As in other tissues, Langerhans cells in the epithelium take up antigens from the microbes, and present them to the immune system, leading to movement of white blood cells into the affected tissues. This process in turn activates osteoclasts which begin to destroy bone, and it activates matrix metalloproteinases that destroy ligaments.[64] So, in summary, it is bacteria which initiates the disease, but key destructive events are brought about by the exaggerated response from the host's immune system.[58]
Classification

There were several attempts to introduce an agreed-upon classification system for periodontal diseases: in 1989, 1993, 1999,[65] and 2017.
1999 classification
The 1999 classification system for periodontal diseases and conditions listed seven major categories of periodontal diseases,[65] of which 2–6 are termed destructive periodontal disease, because the damage is essentially irreversible. The seven categories are as follows:
- Gingivitis
- Chronic periodontitis
- Aggressive periodontitis
- Periodontitis as a manifestation of systemic disease
- Necrotizing ulcerative gingivitis/periodontitis
- Abscesses of the periodontium
- Combined periodontic-endodontic lesions
Moreover, terminology expressing both the extent and severity of periodontal diseases are appended to the terms above to denote the specific diagnosis of a particular person or group of people.
Severity
The "severity" of disease refers to the amount of periodontal ligament fibers that have been lost, termed "clinical attachment loss". According to the 1999 classification, the severity of chronic periodontitis is graded as follows:[66]
- Slight: 1–2 mm (0.039–0.079 in) of attachment loss
- Moderate: 3–4 mm (0.12–0.16 in) of attachment loss
- Severe: ≥ 5 mm (0.20 in) of attachment loss
Extent
The "extent" of disease refers to the proportion of the dentition affected by the disease in terms of percentage of sites. Sites are defined as the positions at which probing measurements are taken around each tooth and, generally, six probing sites around each tooth are recorded, as follows:
- Mesiobuccal
- Mid-buccal
- Distobuccal
- Mesiolingual
- Mid-lingual
- Distolingual
If up to 30% of sites in the mouth are affected, the manifestation is classified as "localized"; for more than 30%, the term "generalized" is used.
2017 classification
The 2017 classification of periodontal diseases is as follows:[67][68][69]
Periodontal health, gingival disease and conditions
- Periodontal health and gingival health
- Clinical gingival health on an intact periodontium
- Clinical gingival health on an intact periodontium
- Stable periodontitis
- Non periodontitis person
- Gingivitis - Dental biofilm induced
- Associated with the dental biofilm alone
- Mediated by systemic and local risk factors
- Drug induced gingival enlargement.
- Gingival diseases - Non dental biofilm induced
- Genetic/developmental disorders
- Specific infections
- Inflammatory and immune conditions
- Reactive processes
- Neoplasms
- Endocrine, nutritional and metabolic
- Traumatic lesions
- Gingival pigmentation.
Periodontitis
- Necrotizing periodontal diseases
- Necrotizing Gingivitis
- Necrotizing periodontitis
- Necrotizing stomatitis
- Periodontitis as a manifestation of systemic disease
- Periodontitis
Other conditions affecting the periodontium
(Periodontal Manifestations of Systemic Diseases and Developmental and Acquired Conditions)
- Systemic disease of conditions affecting the periodontal support tissues
- Other Periodontal Conditions
- Mucogingival deformities and conditions
- Gingival Phenotype
- Gingival/Soft Tissue Recession
- Lack of Gingiva
- Decreased Vestibular Depth
- Aberrant Frenum/muscle position
- Gingival Excess
- Abnormal Color
- Condition of the exposed root surface
- Traumatic occlusal forces
- Primary Occlusal Trauma
- Secondary Occlusal Trauma
- Tooth and prosthesis related factors
- Localized tooth-related factors
- Localized dental prostheses-related factors
Peri-implant diseases and conditions
- Peri-implant health
- Peri-implant mucositis
- Peri-implantitis
- Peri-implant soft and hard tissue deficiencies
Staging
The goals of staging periodontitis is to classify the severity of damage and assess specific factors that may affect management.[69]
According to the 2017 classification, periodontitis is divided into four stages; after considering a few factors such as:
- Amount and percentage bone loss radiographically
- Clinical attachment loss, probing depth
- Presence of furcation
- Vertical bony defects
- History of tooth loss related to periodontitis
- Tooth hypermobility due to secondary occlusal trauma[69]
Grading
According to the 2017 classification, the grading system for periodontitis consists of three grades:[70]
- Grade A: Slow progression of disease; no evidence of bone loss over last five years
- Grade B: Moderate progression; < 2mm of bone loss over last five years
- Grade C: Rapid progression or future progression at high risk; ≥ 2mm bone loss over five years
Risk factors affecting which grade a person is classified into include:[70]
- Smoking
- Diabetes
Prevention
Daily oral hygiene measures to prevent periodontal disease include:
- Brushing properly on a regular basis (at least twice daily), with the person attempting to direct the toothbrush bristles underneath the gumline, helps disrupt the bacterial-mycotic growth and formation of subgingival plaque.[citation needed]
- Flossing daily and using interdental brushes (if the space between teeth is large enough), as well as cleaning behind the last tooth, the third molar, in each quarter.[citation needed]
- Using an antiseptic mouthwash: Chlorhexidine gluconate-based mouthwash in combination with careful oral hygiene may cure gingivitis, although they cannot reverse any attachment loss due to periodontitis.[citation needed]
- Regular dental check-ups and professional teeth cleaning as required: Dental check-ups serve to monitor the person's oral hygiene methods and levels of attachment around teeth, identify any early signs of periodontitis, and monitor response to treatment.
Typically, dental hygienists (or dentists) use special instruments to clean (debride) teeth below the gumline and disrupt any plaque growing below the gumline. This is a standard treatment to prevent any further progress of established periodontitis. Studies show that after such a professional cleaning (periodontal debridement), microbial plaque tends to grow back to precleaning levels after about three to four months. Nonetheless, the continued stabilization of a person's periodontal state depends largely, if not primarily, on the person's oral hygiene at home, as well as on the go. Without daily oral hygiene, periodontal disease will not be overcome, especially if the person has a history of extensive periodontal disease.[citation needed]
Management
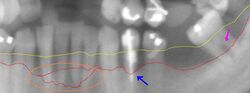
The cornerstone of successful periodontal treatment starts with establishing excellent oral hygiene. This includes twice-daily brushing with daily flossing. Also, the use of an interdental brush is helpful if space between the teeth allows. For smaller spaces, products such as narrow picks with soft rubber bristles provide excellent manual cleaning. Persons with dexterity problems, such as with arthritis, may find oral hygiene to be difficult and may require more frequent professional care and/or the use of a powered toothbrush. Persons with periodontitis must realize it is a chronic inflammatory disease and a lifelong regimen of excellent hygiene and professional maintenance care with a dentist/hygienist or periodontist is required to maintain affected teeth.
Initial therapy
Removal of microbial plaque and calculus is necessary to establish periodontal health. The first step in the treatment of periodontitis involves nonsurgical cleaning below the gum line with a procedure called "root surface instrumentation" or "RSI", this causes a mechanical disturbance to the bacterial biofilm below the gumline.[58] This procedure involves the use of specialized curettes to mechanically remove plaque and calculus from below the gumline, and may require multiple visits and local anesthesia to adequately complete. In addition to initial RSI, it may also be necessary to adjust the occlusion (bite) to prevent excessive force on teeth that have reduced bone support. Also, it may be necessary to complete any other dental needs, such as replacement of rough, plaque-retentive restorations, closure of open contacts between teeth, and any other requirements diagnosed at the initial evaluation. It is important to note that RSI is different to scaling and root planing: RSI only removes the calculus, while scaling and root planing removes the calculus as well as underlying softened dentine, which leaves behind a smooth and glassy surface, which is not a requisite for periodontal healing. Therefore, RSI is now advocated over root planing.[58]
Reevaluation
Nonsurgical scaling and root planing are usually successful if the periodontal pockets are shallower than 4–5 mm (0.16–0.20 in).[71][72][73] The dentist or hygienist must perform a re-evaluation four to six weeks after the initial scaling and root planing, to determine if the person's oral hygiene has improved and inflammation has regressed. Probing should be avoided then, and an analysis by gingival index should determine the presence or absence of inflammation. The monthly reevaluation of periodontal therapy should involve periodontal charting as a better indication of the success of treatment, and to see if other courses of treatment can be identified. Pocket depths of greater than 5–6 mm (0.20–0.24 in) which remain after initial therapy, with bleeding upon probing, indicate continued active disease and will very likely lead to further bone loss over time. This is especially true in molar tooth sites where furcations (areas between the roots) have been exposed.
Surgery
If nonsurgical therapy is found to have been unsuccessful in managing signs of disease activity, periodontal surgery may be needed to stop progressive bone loss and regenerate lost bone where possible. Many surgical approaches are used in the treatment of advanced periodontitis, including open flap debridement and osseous surgery, as well as guided tissue regeneration and bone grafting. The goal of periodontal surgery is access for definitive calculus removal and surgical management of bony irregularities which have resulted from the disease process to reduce pockets as much as possible. Long-term studies have shown, in moderate to advanced periodontitis, surgically treated cases often have less further breakdown over time and, when coupled with a regular post-treatment maintenance regimen, are successful in nearly halting tooth loss in nearly 85% of diagnosed people.[74][75]
Local drug delivery
Local drug deliveries in periodontology has gained acceptance and popularity compared to systemic drugs due to decreased risk in development of resistant flora and other side effects.[76] A meta analysis of local tetracycline found improvement.[77] Local application of statin may be useful.[78]
Systemic drug delivery
Systemic drug delivery in conjunction with non-surgical therapy may be used as a means to reduce the percentage of the bacterial plaque load in the mouth. Many different antibiotics and also combinations of them have been tested; however, there is yet very low-certainty evidence of any significant difference in the short and long term compared to non-surgical therapy alone. It may be beneficial to limit the use of systemic drugs, since bacteria can develop antimicrobial resistance and some specific antibiotics might induce temporary mild adverse effects, such as nausea, diarrhoea and gastrointestinal disturbances.[79]
Adjunctive systemic antimicrobial treatment
There is currently low-quality evidence suggesting if adjunctive systemic antimicrobials are beneficial for the non-surgical treatment of periodontitis.[80] It is not sure whether some antibiotics are better than others when used alongside scaling and root planing).
Maintenance
Once successful periodontal treatment has been completed, with or without surgery, an ongoing regimen of "periodontal maintenance" is required. This involves regular checkups and detailed cleanings every three months to prevent repopulation of periodontitis-causing microorganisms, and to closely monitor affected teeth so early treatment can be rendered if the disease recurs. Usually, periodontal disease exists due to poor plaque control resulting from inappropriate brushing. Therefore, if the brushing techniques are not modified, a periodontal recurrence is probable.
Other
Most alternative "at-home" gum disease treatments involve injecting antimicrobial solutions, such as hydrogen peroxide, into periodontal pockets via slender applicators or oral irrigators. This process disrupts anaerobic micro-organism colonies and is effective at reducing infections and inflammation when used daily. A number of other products, functionally equivalent to hydrogen peroxide, are commercially available, but at substantially higher cost. However, such treatments do not address calculus formations, and so are short-lived, as anaerobic microbial colonies quickly regenerate in and around calculus.
Doxycycline may be given alongside the primary therapy of scaling (see § initial therapy).[81] Doxycycline has been shown to improve indicators of disease progression (namely probing depth and attachment level).[81] Its mechanism of action involves inhibition of matrix metalloproteinases (such as collagenase), which degrade the teeth's supporting tissues (periodontium) under inflammatory conditions.[81] To avoid killing beneficial oral microbes, only small doses of doxycycline (20 mg) are used.[81]
Phage therapy may be a new therapeutic alternative.[82]
Prognosis
Dentists and dental hygienists measure periodontal disease using a device called a periodontal probe. This thin "measuring stick" is gently placed into the space between the gums and the teeth, and slipped below the gumline. If the probe can slip more than 3 mm (0.12 in) below the gumline, the person is said to have a gingival pocket if no migration of the epithelial attachment has occurred or a periodontal pocket if apical migration has occurred. This is somewhat of a misnomer, as any depth is, in essence, a pocket, which in turn is defined by its depth, i.e., a 2-mm pocket or a 6-mm pocket. However, pockets are generally accepted as self-cleansable (at home, by the person, with a toothbrush) if they are 3 mm or less in depth. This is important because if a pocket is deeper than 3 mm around the tooth, at-home care will not be sufficient to cleanse the pocket, and professional care should be sought. When the pocket depths reach 6 to 7 mm (0.24 to 0.28 in) in depth, the hand instruments and ultrasonic scalers used by the dental professionals may not reach deeply enough into the pocket to clean out the microbial plaque that causes gingival inflammation. In such a situation, the bone or the gums around that tooth should be surgically altered or it will always have inflammation which will likely result in more bone loss around that tooth. An additional way to stop the inflammation would be for the person to receive subgingival antibiotics (such as minocycline) or undergo some form of gingival surgery to access the depths of the pockets and perhaps even change the pocket depths so they become 3 mm or less in depth and can once again be properly cleaned by the person at home with his or her toothbrush.
If people have 7-mm or deeper pockets around their teeth, then they would likely risk eventual tooth loss over the years. If this periodontal condition is not identified and people remain unaware of the progressive nature of the disease, then years later, they may be surprised that some teeth will gradually become loose and may need to be extracted, sometimes due to a severe infection or even pain.
According to the Sri Lankan tea laborer study, in the absence of any oral hygiene activity, approximately 10% will experience severe periodontal disease with rapid loss of attachment (>2 mm/year). About 80% will experience moderate loss (1–2 mm/year) and the remaining 10% will not experience any loss.[83][84]
Epidemiology

| no data <3.5 3.5–4 4–4.5 4.5–5 5–5.5 5.5–6 | 6–6.5 6.5–7 7–7.5 7.5–8 8–8.5 >8.5 |
Periodontitis is very common, and is widely regarded as the second most common dental disease worldwide, after dental decay, and in the United States has a prevalence of 30–50% of the population, but only about 10% have severe forms.
Chronic periodontitis affects about 750 million people or about 10.8% of the world population as of 2010.[86]
Like other conditions intimately related to access to hygiene and basic medical monitoring and care, periodontitis tends to be more common in economically disadvantaged populations or regions. Its occurrence decreases with a higher standard of living. In Israeli populations, individuals of Yemenite, North-African, South Asian, or Mediterranean origin have higher prevalence of periodontal disease than individuals from European descent.[87] Periodontitis is frequently reported to be socially patterned, i.e. people from the lower end of the socioeconomic scale are affected more often than people from the upper end of the socioeconomic scale.[60]
History
An ancient hominid from 3 million years ago had gum disease.[88] Records from China and the Middle East, along with archaeological studies, show that mankind has had periodontal disease for at least many thousands of years. In Europe and the Middle East archaeological research looking at ancient plaque DNA, shows that in the ancient hunter-gatherer lifestyle there was less gum disease, but that it became more common when more cereals were eaten. The Otzi Iceman was shown to have had severe gum disease.[citation needed] Furthermore, research has shown that in the Roman era in the UK, there was less periodontal disease than in modern times. The researchers suggest that smoking may be a key to this.[89]
Society and culture
Etymology
The word "periodontitis" (Greek: περιοδοντίτις) comes from the Greek peri, "around", Script error: The function "transl" does not exist. (GEN Script error: The function "transl" does not exist.), "tooth", and the suffix Script error: The function "transl" does not exist., in medical terminology "inflammation".[90] The word pyorrhea (alternative spelling: pyorrhoea) comes from the Greek Script error: The function "transl" does not exist. (πυόρροια), "discharge of matter", itself from Script error: The function "transl" does not exist., "discharge from a sore", rhoē, "flow", and the suffix -ia.[91] In English this term can describe, as in Greek, any discharge of pus; i.e. it is not restricted to these diseases of the teeth.[92]
Economics
It is estimated that lost productivity due to severe periodontitis costs the global economy about US$54 billion each year.[93]
Other animals
Periodontal disease is the most common disease found in dogs and affects more than 80% of dogs aged three years or older. Its prevalence in dogs increases with age, but decreases with increasing body weight; i.e., toy and miniature breeds are more severely affected. Recent research undertaken at the Waltham Centre for Pet Nutrition has established that the bacteria associated with gum disease in dogs are not the same as in humans.[94] Systemic disease may develop because the gums are very vascular (have a good blood supply). The blood stream carries these anaerobic micro-organisms, and they are filtered out by the kidneys and liver, where they may colonize and create microabscesses. The microorganisms traveling through the blood may also attach to the heart valves, causing vegetative infective endocarditis (infected heart valves). Additional diseases that may result from periodontitis include chronic bronchitis and pulmonary fibrosis.[95]
Footnotes
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Gum Disease". February 2018. https://www.nidcr.nih.gov/health-info/gum-disease/more-info.
- ↑ "Gum Disease Complications". https://www.nhs.uk/conditions/gum-disease/complications/.
- ↑ Page, RC; Schroeder, HE (1976). "Pathogenesis of inflammatory periodontal disease. A summary of current work". Laboratory Investigation 34 (3): 235–49. PMID 765622.
- ↑ 4.0 4.1 Albandar, Jasim M.; Adensaya, Margo R.; Streckfus, Charles F.; Winn, Deborah M. (December 2000). "Cigar, Pipe, and Cigarette Smoking as Risk Factors for Periodontal Disease and Tooth Loss". Journal of Periodontology (American Academy of Periodontology) 71 (12): 1874–1881. doi:10.1902/jop.2000.71.12.1874. ISSN 0022-3492. PMID 11156044.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 "Periodontal Disease". 10 July 2013. https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html.
- ↑ 6.0 6.1 ((GBD 2015 Disease and Injury Incidence and Prevalence Collaborators)) (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMID 27733282.
- ↑ 7.0 7.1 V. Baelum and R. Lopez, “Periodontal epidemiology: towards social science or molecular biology?,”Community Dentistry and Oral Epidemiology, vol. 32, no. 4, pp. 239–249, 2004.
- ↑ 8.0 8.1 Nicchio I, Cirelli T, Nepomuceno R, et al. Polymorphisms in Genes of Lipid Metabolism Are Associated with Type 2 Diabetes Mellitus and Periodontitis, as Comorbidities, and with the Subjects' Periodontal, Glycemic, and Lipid Profiles Journal of Diabetes Research. 2021 Jan;2021. PMCID: PMC8601849.
- ↑ "A systematic review of definitions of periodontitis and methods that have been used to identify this disease". Journal of Clinical Periodontology 36 (6): 458–67. June 2009. doi:10.1111/j.1600-051X.2009.01408.x. PMID 19508246.
- ↑ "Gum Disease Treatment". https://www.nhs.uk/conditions/gum-disease/treatment/.
- ↑ 11.0 11.1 11.2 Sáenz-Ravello, Gustavo; Matamala, Loreto; dos Santos, Nidia Castro; Cisternas, Patricia; Gamonal, Jorge; Fernandez, Alejandra; Bello-Escamilla, Natalia; Hernandez, Marcela et al. (1 June 2022). "Healthy Dietary Patterns on Clinical Periodontal Parameters: A GRADE Compliant Systematic Review and Meta-analysis" (in en). Current Oral Health Reports 9 (2): 32–55. doi:10.1007/s40496-022-00307-y. ISSN 2196-3002. https://doi.org/10.1007/s40496-022-00307-y.
- ↑ Sanz, Mariano et al. (2020). "Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline". Journal of Clinical Periodontology 47 (Suppl 22): 4–60. doi:10.1111/jcpe.13290. PMID 32383274.
- ↑ "Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers". Journal of Dental Research 83 (2): 156–60. February 2004. doi:10.1177/154405910408300214. PMID 14742655.
- ↑ "Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study". Journal of Clinical Periodontology 34 (11): 931–7. November 2007. doi:10.1111/j.1600-051X.2007.01133.x. PMID 17877746.
- ↑ "A systematic review and meta-analyses on C-reactive protein in relation to periodontitis". Journal of Clinical Periodontology 35 (4): 277–90. April 2008. doi:10.1111/j.1600-051X.2007.01173.x. PMID 18294231.
- ↑ "Periodontal disease and C-reactive protein-associated cardiovascular risk". Journal of Periodontal Research 39 (4): 236–41. August 2004. doi:10.1111/j.1600-0765.2004.00731.x. PMID 15206916.
- ↑ "Systemic exposure to Porphyromonas gingivalis predicts incident stroke". Atherosclerosis 193 (1): 222–8. July 2007. doi:10.1016/j.atherosclerosis.2006.06.027. PMID 16872615.
- ↑ "Antibodies to periodontal pathogens and stroke risk". Stroke 35 (9): 2020–3. September 2004. doi:10.1161/01.STR.0000136148.29490.fe. PMID 15232116.
- ↑ "High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction". European Journal of Cardiovascular Prevention and Rehabilitation 11 (5): 408–11. October 2004. doi:10.1097/01.hjr.0000129745.38217.39. PMID 15616414.
- ↑ "Anti-P. gingivalis response correlates with atherosclerosis". Journal of Dental Research 86 (1): 35–40. January 2007. doi:10.1177/154405910708600105. PMID 17189460.
- ↑ "Periodontal disease and coronary heart disease: a reappraisal of the exposure". Circulation 112 (1): 19–24. July 2005. doi:10.1161/CIRCULATIONAHA.104.511998. PMID 15983248.
- ↑ "Associations between periodontal disease and risk for atherosclerosis, cardiovascular disease, and stroke. A systematic review". Annals of Periodontology 8 (1): 38–53. December 2003. doi:10.1902/annals.2003.8.1.38. PMID 14971247.
- ↑ "Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study". Archives of Internal Medicine 160 (18): 2749–55. October 2000. doi:10.1001/archinte.160.18.2749. PMID 11025784.
- ↑ "Relationship of periodontal disease to carotid artery intima-media wall thickness: the atherosclerosis risk in communities (ARIC) study". Arteriosclerosis, Thrombosis, and Vascular Biology 21 (11): 1816–22. November 2001. doi:10.1161/hq1101.097803. PMID 11701471.
- ↑ "Relationship of periodontal disease and tooth loss to prevalence of coronary heart disease". Journal of Periodontology 75 (6): 782–90. June 2004. doi:10.1902/jop.2004.75.6.782. PMID 15295942.
- ↑ "Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis". Journal of General Internal Medicine 23 (12): 2079–86. December 2008. doi:10.1007/s11606-008-0787-6. PMID 18807098.
- ↑ "Association between periodontitis and arterial hypertension: A systematic review and meta-analysis". American Heart Journal 180: 98–112. October 2016. doi:10.1016/j.ahj.2016.07.018. PMID 27659888.
- ↑ "Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III". Journal of Neurology, Neurosurgery, and Psychiatry 80 (11): 1206–11. November 2009. doi:10.1136/jnnp.2009.174029. PMID 19419981.
- ↑ "Tooth loss and periodontal disease predict poor cognitive function in older men". Journal of the American Geriatrics Society 58 (4): 713–8. April 2010. doi:10.1111/j.1532-5415.2010.02788.x. PMID 20398152.
- ↑ "Periodontal disease might be associated even with impaired fasting glucose". British Dental Journal 208 (10): E20. May 2010. doi:10.1038/sj.bdj.2010.291. PMID 20339371.
- ↑ "The relationship between periodontal diseases and diabetes: an overview". Annals of Periodontology 6 (1): 91–8. December 2001. doi:10.1902/annals.2001.6.1.91. PMID 11887477.
- ↑ "Erectile dysfunction might be associated with chronic periodontal disease: two ends of the cardiovascular spectrum". The Journal of Sexual Medicine 6 (4): 1111–1116. April 2009. doi:10.1111/j.1743-6109.2008.01141.x. PMID 19170861.
- ↑ Poyato-Borrego, M.; Segura-Egea, J.-J.; Martín-González, J.; Jiménez-Sánchez, M.-C.; Cabanillas-Balsera, D.; Areal-Quecuty, V.; Segura-Sampedro, J.-J. (27 August 2020). "Prevalence of endodontic infection in patients with Crohn´s disease and ulcerative colitis". Medicina Oral, Patologia Oral y Cirugia Bucal 26 (2): e208–e215. doi:10.4317/medoral.24135. ISSN 1698-6946. PMID 32851982.
- ↑ Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M. (3 May 2012). "European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR)". European Heart Journal 33 (13): 1635–1701. doi:10.1093/eurheartj/ehs092. PMID 22555213.
- ↑ Michaud, Dominique S.; Izard, Jacques (2014). "Microbiota, Oral Microbiome, and Pancreatic Cancer". The Cancer Journal 20 (3): 203–206. doi:10.1097/PPO.0000000000000046. PMID 24855008.
- ↑ Nascimento, Gustavo G.; Leite, Fábio R. M.; Vestergaard, Peter; Scheutz, Flemming; López, Rodrigo (July 2018). "Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies". Acta Diabetologica 55 (7): 653–667. doi:10.1007/s00592-018-1120-4. ISSN 1432-5233. PMID 29502214. https://pubmed.ncbi.nlm.nih.gov/29502214/.
- ↑ Michaud, Dominique S.; Fu, Zhuxuan; Shi, Jian; Chung, Mei (January 2017). "Periodontal Disease, Tooth Loss, and Cancer Risk". Epidemiologic Reviews 39 (1): 49–58. doi:10.1093/epirev/mxx006. ISSN 0193-936X. PMID 28449041.
- ↑ Gomes-Filho, Isaac Suzart; Freitas Coelho, Julita Maria; da Cruz, Simone Seixas; Passos, Johelle Santana; Teixeira de Freitas, Camila Oliveira; Aragão Farias, Naiara Silva; Amorim da Silva, Ruany; Silva Pereira, Milena Novais et al. (July 2011). "Chronic periodontitis and C-reactive protein levels". Journal of Periodontology 82 (7): 969–978. doi:10.1902/jop.2010.100511. ISSN 1943-3670. PMID 21189085. https://pubmed.ncbi.nlm.nih.gov/21189085/.
- ↑ Loos, Bruno G. (November 2005). "Systemic markers of inflammation in periodontitis". Journal of Periodontology 76 (11 Suppl): 2106–2115. doi:10.1902/jop.2005.76.11-S.2106. ISSN 0022-3492. PMID 16277583. https://pubmed.ncbi.nlm.nih.gov/16277583/.
- ↑ Libby, Peter; Ridker, Paul M.; Hansson, Göran K.; Leducq Transatlantic Network on Atherothrombosis (2009-12-01). "Inflammation in atherosclerosis: from pathophysiology to practice". Journal of the American College of Cardiology 54 (23): 2129–2138. doi:10.1016/j.jacc.2009.09.009. ISSN 1558-3597. PMID 19942084.
- ↑ Harada, Masahide; Van Wagoner, David R.; Nattel, Stanley (2015). "Role of Inflammation in Atrial Fibrillation Pathophysiology and Management". Circulation Journal 79 (3): 495–502. doi:10.1253/circj.cj-15-0138. ISSN 1346-9843. PMID 25746525. PMC 4457364. http://dx.doi.org/10.1253/circj.cj-15-0138.
- ↑ Tsutsui, Hiroyuki; Kinugawa, Shintaro; Matsushima, Shouji (December 2011). "Oxidative stress and heart failure". American Journal of Physiology. Heart and Circulatory Physiology 301 (6): H2181–2190. doi:10.1152/ajpheart.00554.2011. ISSN 1522-1539. PMID 21949114. https://pubmed.ncbi.nlm.nih.gov/21949114/.
- ↑ Marouf, Nadya (2021). "Association between periodontitis and severity of COVID-19 infection: A case–control study". Journal of Clinical Periodontology 48 (4): 483–491. doi:10.1111/jcpe.13435. PMID 33527378.
- ↑ Najeeb, Shariq; Zafar, Muhammad Sohail; Khurshid, Zohaib; Zohaib, Sana; Almas, Khalid (30 August 2016). "The Role of Nutrition in Periodontal Health: An Update". Nutrients 8 (9): 530. doi:10.3390/nu8090530. PMID 27589794.
- ↑ "Gingivitis". Rochester, Minnesota: MFMER. 4 August 2017. https://www.mayoclinic.org/diseases-conditions/gingivitis/symptoms-causes/syc-20354453.
- ↑ "Blastomycosis of the Gingiva and Jaw". Canadian Medical Association Journal 26 (6): 662–5. June 1932. PMID 20318753.
- ↑ "Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets". Medical Mycology 46 (8): 783–93. December 2008. doi:10.1080/13693780802060899. PMID 18608938.
- ↑ "Endogenous Aspergillus endophthalmitis associated with periodontitis". Ophthalmologica. Journal International d'Ophtalmologie. International Journal of Ophthalmology. Zeitschrift für Augenheilkunde 209 (2): 109–11. 1995. doi:10.1159/000310592. PMID 7746643.
- ↑ "Periodontal aspects of the juvenile form of paracoccidioidomycosis". Revista do Instituto de Medicina Tropical de Sao Paulo 40 (1): 15–8. 1998. doi:10.1590/S0036-46651998000100004. PMID 9713132.
- ↑ "Diabetes mellitus promotes periodontal destruction in children". Journal of Clinical Periodontology 34 (4): 294–8. April 2007. doi:10.1111/j.1600-051X.2007.01054.x. PMID 17378885.
- ↑ "Diabetes and Periodontal Disease". https://www.webmd.com/diabetes/periodontal-disease#1.
- ↑ "Effects of smoking on periodontal health: a review". Advances in Therapy 17 (5): 230–7. 2000. doi:10.1007/BF02853162. PMID 11186143.
- ↑ "Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey". Journal of Periodontology 71 (5): 743–51. May 2000. doi:10.1902/jop.2000.71.5.743. PMID 10872955.
- ↑ "The influence of smoking on host responses in periodontal infections". Periodontology 2000 43 (1): 267–77. 2007. doi:10.1111/j.1600-0757.2006.00163.x. PMID 17214844.
- ↑ "Effect of cigarette smoking on oral elastase activity in adult periodontitis patients". Journal of Periodontology 71 (1): 58–62. January 2000. doi:10.1902/jop.2000.71.1.58. PMID 10695939.
- ↑ "Effect of tobacco smoking on neutrophil activity following periodontal surgery". Journal of Periodontology 74 (10): 1475–82. October 2003. doi:10.1902/jop.2003.74.10.1475. PMID 14653394.
- ↑ "Tobacco smoking and periodontal hemorrhagic responsiveness". Journal of Clinical Periodontology 28 (7): 680–5. July 2001. doi:10.1034/j.1600-051x.2001.028007680.x. PMID 11422590.
- ↑ 58.0 58.1 58.2 58.3 58.4 58.5 58.6 "Current Concepts in Periodontal Pathogenesis". DentalUpdate 31 (10): 570–578. December 2004. https://www.dental-update.co.uk/issuesThreeArticle.asp?aKey=421.
- ↑ "A systematic review of stress and psychological factors as possible risk factors for periodontal disease". Journal of Periodontology 78 (8): 1491–504. August 2007. doi:10.1902/jop.2007.060371. PMID 17668968.
- ↑ 60.0 60.1 Watt, RG; Listl, S; Peres, MA et al., eds (2015). Social inequalities in oral health: from evidence to action. London: UCL. http://media.news.health.ufl.edu/misc/cod-oralhealth/docs/posts_frontpage/SocialInequalities.pdf.
- ↑ 61.0 61.1 "Periodontitis as a possible early sign of diabetes mellitus". BMJ Open Diabetes Research & Care 5 (1): e000326. 1 January 2017. doi:10.1136/bmjdrc-2016-000326. PMID 28316794.
- ↑ 62.0 62.1 62.2 "Diabetes and periodontal disease: a two-way relationship". British Dental Journal 217 (8): 433–7. October 2014. doi:10.1038/sj.bdj.2014.907. PMID 25342350.
- ↑ "A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes". Journal of Clinical Periodontology 40 (Suppl 14): S113–34. April 2013. doi:10.1111/jcpe.12059. PMID 23627323.
- ↑ 64.0 64.1 64.2 64.3 "Periodontal diseases". Nature Reviews. Disease Primers 3: 17038. June 2017. doi:10.1038/nrdp.2017.38. PMID 28805207.
- ↑ 65.0 65.1 "Development of a classification system for periodontal diseases and conditions". Annals of Periodontology 4 (1): 1–6. December 1999. doi:10.1902/annals.1999.4.1.1. PMID 10863370.
- ↑ "The Periodontal Disease Classification System of the American Academy of Periodontology – An Update". http://www.cda-adc.ca/jcda/vol-66/issue-11/594.html.
- ↑ "Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions". Journal of Clinical Periodontology 45 (Suppl 20): S219–S229. June 2018. doi:10.1111/jcpe.12951. PMID 29926500. https://research-information.bristol.ac.uk/files/161189168/R2corr_Workgroup3_Consensus_Report.pdf.
- ↑ "Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions". Journal of Clinical Periodontology 45 (Suppl 20): S286–S291. June 2018. doi:10.1111/jcpe.12957. PMID 29926491.
- ↑ 69.0 69.1 69.2 "A new classification scheme for periodontal and peri-implant diseases and conditions – Introduction and key changes from the 1999 classification". Journal of Periodontology 89 (Suppl 1): S1–S8. June 2018. doi:10.1002/jper.18-0157. PMID 29926946. http://pure-oai.bham.ac.uk/ws/files/50172936/Caton_et_al_Version_3.0_NO_TABLES_PNP_amendments_1_ILC.pdf.
- ↑ 70.0 70.1 "Staging and Grading Periodontitis". http://perio.org/sites/default/files/files/Staging%20and%20Grading%20Periodontitis.pdf.
- ↑ "The limits of subgingival scaling". The International Journal of Periodontics & Restorative Dentistry 1 (5): 30–41. 1981. PMID 7047434.
- ↑ "Healing of the dento-epithelial junction following subgingival plaque control. I. As observed in human biopsy material". Journal of Periodontology 49 (1): 1–8. January 1978. doi:10.1902/jop.1978.49.1.1. PMID 340634.
- ↑ "Healing of the dento-epithelial junction following subgingival plaque control. II: As observed on extracted teeth". Journal of Periodontology 49 (3): 119–34. March 1978. doi:10.1902/jop.1978.49.3.119. PMID 288899.
- ↑ "Long-term evaluation of periodontal therapy: II. Incidence of sites breaking down". Journal of Periodontology 67 (2): 103–8. February 1996. doi:10.1902/jop.1996.67.2.103. PMID 8667129.
- ↑ "A long-term survey of tooth loss in 600 treated periodontal patients". Journal of Periodontology 49 (5): 225–37. May 1978. doi:10.1902/jop.1978.49.5.225. PMID 277674.
- ↑ "Effect of local drug delivery in chronic periodontitis patients: A meta-analysis". Journal of Indian Society of Periodontology 15 (4): 304–9. October 2011. doi:10.4103/0972-124X.92559. PMID 22368351.
- ↑ "Tetracycline as local drug delivery in treatment of chronic periodontitis: A systematic review and meta-analysis". Journal of Indian Society of Periodontology 20 (6): 576–583. 2016. doi:10.4103/jisp.jisp_97_17. PMID 29238136.
- ↑ "The effect of local and systemic statin use as an adjunct to non-surgical and surgical periodontal therapy-A systematic review and meta-analysis". Journal of Dentistry 67: 18–28. December 2017. doi:10.1016/j.jdent.2017.08.011. PMID 28855141. http://urn.kb.se/resolve?urn=urn:nbn:se:mau:diva-16218.
- ↑ "Adjunctive systemic antimicrobials for the non-surgical treatment of periodontitis.". Cochrane Database of Systematic Reviews 2020 (11): 1465–1858. 2020. doi:10.1002/14651858.CD012568.pub2. CD012568. PMID 33197289.
- ↑ Khattri, Shivi; Kumbargere Nagraj, Sumanth; Arora, Ankita; Eachempati, Prashanti; Kusum, Chandan Kumar; Bhat, Kishore G; Johnson, Trevor M; Lodi, Giovanni (2020-11-16). "Adjunctive systemic antimicrobials for the non-surgical treatment of periodontitis". Cochrane Database of Systematic Reviews 2020 (11): CD012568. doi:10.1002/14651858.cd012568.pub2. ISSN 1465-1858. PMID 33197289. PMC 9166531. https://doi.org/10.1002/14651858.CD012568.pub2.
- ↑ 81.0 81.1 81.2 81.3 "Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD)". Pharmacological Research 63 (2): 114–20. February 2011. doi:10.1016/j.phrs.2010.12.003. PMID 21182947.
- ↑ . Liviu Steier, Silvia Dias de Oliveira, José Antonio Poli de Figueiredo"Bacteriophages in Dentistry—State of the Art and Perspectives". Dent J 7 (1): 6. 2019. doi:10.3390/dj7010006. PMID 30634460.
- ↑ "The natural history of periodontal disease. The correlation of selected microbiological parameters with disease severity in Sri Lankan tea workers". Journal of Clinical Periodontology 22 (9): 674–8. September 1995. doi:10.1111/j.1600-051X.1995.tb00825.x. PMID 7593696.
- ↑ "Tooth mortality in plantation workers and residents in Sri Lanka". Community Dentistry and Oral Epidemiology 12 (2): 128–35. April 1984. doi:10.1111/j.1600-0528.1984.tb01425.x. PMID 6584263.
- ↑ "Mortality and Burden of Disease Estimates for WHO Member States in 2002" (xls). World Health Organization. 2002. https://www.who.int/entity/healthinfo/statistics/bodgbddeathdalyestimates.xls.
- ↑ "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet 380 (9859): 2163–96. December 2012. doi:10.1016/S0140-6736(12)61729-2. PMID 23245607.
- ↑ "[Ethnic origin and alveolar bone loss in Israeli adults]" (in he). Refu'at Ha-Peh Veha-Shinayim 25 (2): 19–22, 72. April 2008. PMID 18780541.
- ↑ Forshaw, R. J. (April 2009). "Dental health and disease in ancient Egypt". British Dental Journal 206 (8): 421–424. doi:10.1038/sj.bdj.2009.309. PMID 19396207.
- ↑ "Roman-Britons had less gum disease than modern Britons". 24 October 2014. https://www.heritagedaily.com/2014/10/roman-britons-had-less-gum-disease-than-modern-britons/105351.
- ↑ Harper, Douglas. "periodontitis". Online Etymology Dictionary. https://www.etymonline.com/?term=periodontitis. Harper, Douglas. "periodontal". Online Etymology Dictionary. https://www.etymonline.com/?term=periodontal. ὀδούς, ὀδών. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- ↑ πυόρροια, πύον, ῥοή; cf. πυορροέω in Liddell and Scott. Harper, Douglas. "-ia". Online Etymology Dictionary. https://www.etymonline.com/?term=-ia.
- ↑ "pyorrhea". pyorrhea. http://medical-dictionary.thefreedictionary.com/pyorrhea.
- ↑ "Global Economic Impact of Dental Diseases". Journal of Dental Research 94 (10): 1355–61. October 2015. doi:10.1177/0022034515602879. PMID 26318590.
- ↑ "The canine oral microbiome". PLOS ONE 7 (4): e36067. 2012. doi:10.1371/journal.pone.0036067. PMID 22558330. Bibcode: 2012PLoSO...736067D.
- ↑ Muller-Esnault, Susan (2009). "Periodontal Disease in the Dog and Cat". Critterology. Veterinary Internet Company. http://www.critterology.com/articles/periodontal-disease-dog-and-cat.
External links
| Classification | |
|---|---|
| External resources |
- Canadian Academy of Periodontology — What is periodontitis?
- Periodontal disease and braces. Orthodontics Australia.
 |

