Biology:Mammalian kidney
| Mammalian kidney | |
|---|---|
 Unipapillary, multilobar, smooth, bean-shaped camel kidney, in which the renal papillae are completely fused into the renal crest.[1] | |
| Details | |
| Precursor | Ureteric bud, metanephrogenic blastema |
| System | Urinary system and endocrine system |
| Artery | Renal artery |
| Vein | Renal vein |
| Nerve | Renal plexus |
| Lymph | Collecting lymphatic vessels |
| Anatomical terminology | |
The mammalian kidneys are a pair of excretory organs of the urinary system of mammals. They are a type of metanephric kidney.[2] The kidneys in mammals are usually bean-shaped[3] and located behind the peritoneum (retroperitoneally)[4] on the back (dorsal) wall of the body.[5] Each kidney consists of a renal capsule, peripheral cortex, internal medulla, calices, and renal pelvis, although the calices or renal pelvis may be absent in some species. Urine is excreted from the kidney through the ureter.[5] Nitrogen-containing waste products are excreted by the kidneys in mammals mainly in the form of urea.[6]
The structure of the kidney may differ between species.[7] The kidneys can be unilobar (single lobe) or multilobar, unipapillary (single papilla), with several papillae or multipapillary,[8] may be smooth-surfaced or lobulated,[1][9] also the kidneys may be reniculate, which are found mainly in marine mammals.[10] The simplest type of kidney in mammals is the unipapillary kidney with single lobe.[11][4] Differences in kidney structure are the result of adaptations during evolution to variations in body mass and habitats (in particular, habital aridity) between species[12][13][7]. The human kidney is an example of the mammalian kidney.
The cortex and medulla of the kidney contain nephrons.[14] The cortex contains glomeruli and is responsible for filtering the blood.[5] In mammals, the nephrons can be classified into the nephrons with a short loop and a long loop of Henle,[15] and the loop of Henle is responsible for urine concentration.[16] Amongst the vertebrates, only mammals and birds have kidneys that can produce urine more concentrated (hypertonic) than the blood plasma,[5] but only in mammals do all nephrons have the loop of Henle.[17]
The kidneys of mammals are vital organs[18] that maintain water, electrolyte and acid-base balance in the body, excrete nitrogenous waste products, regulate blood pressure, participate in bone formation[19][20][21] and in regulation of glucose levels.[22] The processes of blood plasma filtration, tubular reabsorption and tubular secretion occur in the kidneys, and urine formation is a result of these processes.[23] The kidneys produce renin[24] and erythropoietin[25] hormones, and are involved in the conversion of vitamin D to its active form.[26] Mammals are the only class of vertebrates in which only the kidneys are responsible for maintaining the homeostasis of the extracellular fluid in the body.[27] The function of the kidneys is regulated by the autonomic nervous system and hormones.[28]
The kidneys can have noninfectious and infectious diseases; in rare cases, congenital and hereditary anomalies occur in the kidneys of mammals.[29] Pyelonephritis is usually caused by bacterial infections.[30][31] Some diseases may be species specific,[32] and parasitic kidney diseases are common in some species.[33][34] The structural characteristics of the mammalian kidneys make them vulnerable to ischemic and toxic injuries.[35] The potential for regeneration in mature kidneys is limited[36][37] because new nephrons cannot be formed,[38] but in cases of limited injury, renal function can be restored through compensatory mechanisms.[39] Permanent damage can lead to chronic kidney disease.[40][41] Ageing of the kidneys also causes changes in them, and the number of functioning nephrons decreases with age.[42]
Structure
Gross anatomy

- 1. Fibrous capsule
- 2. Cortex
- 3. Renal pyramid of the medulla
- 4. Renal column of the cortex
- 5. Nephron
- 6. Renal papilla
- 7. Minor renal calyx
- 8. Major renal calyx
- 9. Renal pelvis
- 10. Ureter
- 11. Renal artery
- 12. Renal vein
- 13. Interlobar artery
- 14. Renal lobe
- 15. Arcuate artery
- 16. Interlobular artery.
Location and shape
In mammals, kidneys are usually bean-shaped;[3] the shape is unique to mammals (fish, for example, has elongated kidneys).[44] Kidneys are located retroperitoneally[4] on the back (dorsal) wall of the body.[5] One of the key factors that determine the shape and morphology of the kidneys in mammals is their mass.[45] The concave part of the bean-shaped kidneys is called the renal hilum, through which the renal artery and nerves enter the kidney. The renal vein, collecting lymphatic vessels and ureter exit the kidney through the renal hilum.[4][46] In the body, the kidney is surrounded by a mass of adipose tissue.[47]
General structure
The outer layer of each kidney is made up of a fibrous sheath called a renal capsule. The peripheral layer of the kidney is called the cortex, and the inner layer is called the medulla. The medulla consists of pyramids (also called malpighian pyramids), ascending with their base to the cortex and forming together with it the renal lobe.[48] The pyramids are separated from each other by renal columns (Bertin's columns) formed by cortical tissue.[49] The tips of the pyramids end with the renal papillae, from which urine is excreted into the calyces, pelvis, ureter, and, in most species, directly into the bladder,[48][50] after which it is excreted through the urethra.[51]
Parenchyma
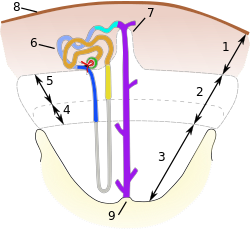
- 1. Cortex
- 2. Outer medulla
- 3. Inner medulla
- 4. Inner stripe
- 5. Outer stripe
- 6. Juxtamedullary nephron
- 7. Medullary ray
- 8. Renal capsule
- 9. Renal papilla.
The parenchyma, being the functional part of the kidneys, is visually divided into cortex and medulla.[53][54] The cortex and medulla are based on nephrons[55][56] together with an extensive network of blood vessels and capillaries, as well as collecting ducts, into which nephrons empty, and renal interstitium.[57] There is a blood-filtering part of the nephron in the cortex — the renal corpuscle, from which the renal tubule extends to the medulla into the loop of Henle, then the tubule returns back to the cortex and with its distal end flows into its collecting duct that is common to several nephrons. The collecting ducts descend again into the medulla and fuse to wider collecting ducts which pass through the inner medulla.[58][59]
Based on the location of the renal corpuscle, nephrons are classified into 3 types: superficial (closer to the renal capsule), midcortical (in the middle part of the cortex) and juxtamedullary (closer to the medulla) nephrons. According to the length of the loop of Henle, nephrons are classified into nephrons with a long loop and with a short loop of Henle.[60]
Cortex
Structurally, the cortex consists of cortical labyrinth and medullary rays.[61] The cortical labyrinth contains interlobular arteries, vascular networks formed by afferent and efferent arterioles, renal corpuscles, proximal convoluted tubules, macula densa, distal convoluted tubules, connecting tubules and the initial parts of the collecting ducts.[62] The proximal convoluted tubules predominate in the cortical labyrinth.[63] The continuous layer of the cortex lying above the medullary rays is called the cortex corticis.[61] Some mammals have nephrons whose loops of Henle do not reach the medulla; such nephrons are called cortical nephrons.[58] The medullary rays of the cortex contain the proximal straight tubules, the cortical part of the thick ascending limb of the loops of Henle, and the cortical part of the collecting ducts.[62] The cortex is divided into lobules, each of which is a medullary ray in conjunction with connected to it nephrons, and interlobular arteries that pass between the lobules.[64] The ratio of cortex to medulla varies between species, in domesticated animals the cortex usually occupies a third or fourth part of the parenchyma, while in desert animals with long loops of Henle it is only a fifth part.[9]
Medulla
The medulla in mammals is divided into outer and inner regions. The outer region consists of short loops of Henle and collecting ducts, while the inner region consists of long loops and collecting ducts.[65] The outer region is also subdivided into outer[66] (lying directly under the cortex)[67] and inner stripes.[66] The stripes differ in that the outer stripe contain proximal straight tubules, while the inner strite contain thin descending limbs of the loop of Henle (a section of the nephron following the proximal straight tubule).[67]
The ratio of the outer and inner medulla
Most mammalian species have nephrons with both short and long loops of Henle, while some species may have only one type. For example, mountain beavers have only nephrons with a short loop, and, accordingly, there is no inner medulla in the kidney. Dogs and cats, on the other hand, have only long-loop nephrons. The ratio of nephrons with short loops of Henle to those with long loops also varies between species.[68]
Structural differences between species

- (A) Equine kidneys with heart-shaped right kidney
- (B) Bovine kidneys with lobulated cortex and fused medulla
- (C) Canine bean-shaped kidneys.
Structurally, kidneys vary between mammals.[70] What structural type a particular species will have depends generally on the body mass of the animals.[71] Small mammals have unilobar kidneys with a compact structure and a single renal papilla,[70][8] while larger animals have multilobar kidneys, such as those of bovines,[70][8] but bovine kidneys are also externally lobulated (visually divided into lobes).[9] By itself, the lobe is equivalent to a simple unipapillary kidney, as in rats or mice.[63] Bovine kidneys also lack renal pelvis, urine from the major calices is excreted directly into the ureter.[72]
Kidneys can be unipapillary,[8] as in rats and mice,[73] with few renal papillae, as in spider monkeys, or with many, as in pigs and humans.[8] Most animals have single renal papilla.[8] In some animals, such as horses, the tips of the renal pyramids fuse with each other to form a common renal papilla, called the renal crest.[74] The renal crest usually appears in animals larger than the rabbit.[75]

- 1. Renal artery
- 2. Renal vein
- 3. Common collecting duct that becomes the ureter.
In marine mammals, otters and bears, the kidneys are reniculate, consisting of small reniculi,[10] each of which is comparable to a simple unilobar kidney.[61] Marine mammal kidneys can have hundreds[10] or thousands[41] of reniculi, each with its own cortex, medulla, and calyx.[10] For example, each whale kidney consist of about 7000 renculi which join a common collective system.[41] In manatees, which are also marine mammals, the kidneys are actually multilobar, since the cortex is continuous.[10]
The size of the kidneys increases with the mass of mammals, and the number of nephrons in the kidneys between mammals increases allometrically.[77] In mice, the kidneys are approximately 1 cm (0.4 in) long, weighing 400 mg, with 16,000 nephrons, while in the killer whale, the kidney length exceeds 25 cm (10 in), the mass is approximately 4.5 kg (10 lb), with the number of nephrons of the order of 10,000,000. At the same time, killer whale kidneys are reniculate, and each reniculus is comparable to the kidney of mice.[78]
Microanatomy
By microanatomic structure, the kidney can be divided into several main elements: interstitium, renal corpuscles, tubules, and vasculature.[9] The interstitium is the cells and extracellular matrix in the space between the glomeruli, vessels, tubules, and collecting ducts.[79][80] The interstitial space surrounding cells is filled with interstitial fluid.[81] The interstitium between the tubules contains fibroblasts, dendritic cells, macrophages and lymphocytes. Cortical interstitium also includes the endothelial cells of the lymphatic capillaries,[81] which are considered part of the interstitium due to the lack of a basement membrane.[82] Interstitial fibroblasts form the tissue skeleton of the kidney.[83] Blood vessels, nerves and lymphatic vessels run through the interstitium.[84] The nephron, together with the collecting duct into which it empties, is called the uriniferous tubule. Each uriniferous tubule, along with the vasculature supplying it, is embedded in the interstitium.[9]
Approximately 18–26 different cell types have been described in mammalian kidneys, with a large variation in the range due to a lack of consensus on what counts as a particular cell type, and likely to species differences.[85] Renal corpuscles are composed of 4 cell types: fenestrated endothelium, mesangial cells, podocytes and parietal epithelial cells of Bowman's capsule.[86] At least 16 different cell types make up the renal tubules.[87] The tubules themselves are divided into at least 14 segments,[87] which differ in cell types and functions.[88] The normal functioning of the kidneys is provided by the complex of epithelial, endothelial, interstitial and immune cells.[89]
Blood supply

Blood enters the kidney through the renal artery,[46] which in the multilobar kidney branches in the area of the renal pelvis into large interlobar arteries that pass through the renal columns.[50][90] The interlobar arteries branch at the base of the pyramid, giving rise to arcuate arteries, from which the interlobular arteries extend into the cortex.[90] The interlobar arteries supply the pyramids and the adjacent cortex with an extensive network of blood vessels.[50] The cortex itself is heavily permeated with arteries, while there are no arteries in the medulla.[11] The venous flow of blood runs back parallel to the arteries.[90] In some species, in the cortex there are veins isolated from the arteries under the capsule, which in humans are called stellate veins. These veins flow into the interlobular veins.[91] The renal portal system is absent in mammals,[92] with the exception of monotremes.[93] Mammals are the only class of vertebrates (with exception of some species) that does not have a renal portal system.[94]
The vascular glomeruli of nephrons receive blood from afferent arterioles, which originate in the interlobular arteries with intermediate formation of prearterioles. Each afferent arteriole divides into several renal glomeruli. Then these glomeruli join into the efferent arteriole, into which filtered blood goes from the nephrons. In nephrons with a long loop of Henle, the efferent arterioles branch, forming straight vessels called vasa recta, descending into the medulla. The descending vasa recta, ascending vasa recta vessels, and the loop of Henle together form the countercurrent system of the kidney. In the afferent arteriole, blood is supplied at high pressure, which promotes filtration, and in the efferent arteriole, it is at low pressure, which promotes reabsorption.[90]
Despite their small size, the kidneys of mammals account for a significant part of the minute volume of blood circulation.[95] It is believed that in land mammals, about a fifth of the volume of blood that passes through the heart passes through the kidneys.[96] In adult mice, for example, minute volume is about 9%–22%.[97]
Lymphatic system
The kidney is well supplied with lymphatic vessels,[98] which remove excess fluid with substances and macromolecules dissolved in it from the interstitium that fills the space between the tubules and blood vessels.[99][100] The anatomy of the lymphatic system of the kidney is similar between mammals.[101] Lymphatics basically follow the path of blood vessels.[102]
The lymphatic system of the kidneys begins in the cortex with the initial blind-end intralobular lymphatic capillaries passing near the tubules and renal corpuscles, but the lymphatic vessels do not go inside the renal corpuscles. The intralobular lymphatic capillaries are connected to the arcuate lymphatics.[103] The arcuate lymphatics pass into the interlobar lymphatics, which pass near the interlobar arteries.[103][101] The arcuate and interlobar lymphatics are lymphatic precollectors.[82] Finally, the interlobar lymphatics join the collecting hilar lymphatics leaving the kidney through renal hilum.[103] Lymphatic vessels are usually absent in the medulla of the mammalian kidneys, and the role of lymphatic vessels is assumed to be performed by vasa recta.[104][105]
In some species, there may be differences in the anatomy of the lymphatic system of the kidney. For example, sheep lack lymphatics in the renal capsule, and rabbits lack interlobular lymphatics.[103] Most studies fail to detect lymphatic vessels in the renal medulla of animals, in particular, they are not found in sheep and rats. But some studies have found lymphatic vessels in the renal medulla of pigs and rabbits.[105] Depending on the species, there may or may not also be a connection between the lymphatics of the renal capsule and the internal renal lymphatic system.[106]
Nerve supply
The innervation of the kidney is provided by efferent sympathetic nerve fibers entering the kidney through the renal hilum,[46] originating in the celiac plexus,[107] and afferent, leaving the kidney to the spinal ganglion.[107] There is no reliable evidence for the innervation of the kidney by parasympathetic nerves,[107] while the existing evidence is controversial.[108] Efferent sympathetic nerve fibers reach the renal vasculature, renal tubules, juxtaglomerular cells, and the wall of the renal pelvis,[109] all parts of the nephron are innervated by sympathetic nerves.[107] Nerve fibers pass through the connective tissue around the arteries and arterioles. In the medulla, the descending vasa recta are innervated as long as they contain smooth muscle cells.[110] Most afferent nerve fibers are located in the renal pelvis.[111] The vast majority of nerves in the kidneys are unmyelinated.[112]
Normal physiological stimulation of the efferent sympathetic nerves of the kidney is involved in maintaining the balance of water and sodium in the body. Activation of the efferent sympathetic nerves of the kidney reduces its blood flow, and respectively, filtration and excretion of sodium in the urine, and also increases the rate of renin secretion.[113] The afferent nerves in the kidney are also involved in maintaining balance. Mechanosensory nerves of the kidney are activated by stretching of the tissue of the renal pelvis, which can occur with an increase in the rate of urine flow from the kidney, resulting in a reflex decrease in the activity of efferent sympathetic nerves. That is, activation of the afferent nerves in the kidney suppresses the activity of the efferent nerves.[114]
Functions
Excretory function
In mammals, nitrogenous metabolic products are excreted predominantly in the form of urea,[6] which is the end by-product of mammalian protein metabolism[115][116] and is highly soluble in water.[117] Most of the urea is excreted by the kidneys.[115] Blood filtration, as in other vertebrates, occurs in the renal glomeruli, where pressurized blood passes through a permeable barrier that filters out blood cells and large protein molecules, forming primary urine. Filtered primary urine is osmotically and ionically the same as blood plasma. In the tubules of the nephron, substances useful for the body, dissolved in the primary urine, are subsequently reabsorbed, as the urine is being concentrated.[118]
Osmoregulation
Mammalian kidneys maintain an almost constant level of plasma osmolarity. The main component of blood plasma, which determines its osmolarity, is sodium and its anions.[119] The key role in maintaining a constant level of osmolarity is managed by the control of the ratio of sodium and water in the blood.[119][120] Drinking large amounts of water can dilute the blood plasma, in which case the kidneys produce more dilute urine than the plasma to keep the salt in the blood but to remove the excess water. If too little water is consumed, then urine is excreted more concentrated than blood plasma.[119] The concentration of urine is provided by an osmotic gradient that increases from the border between the cortex and medulla to the top of the pyramid of the medulla.[119]
In addition to the kidneys, the hypothalamus and neurohypophysis are involved in the regulation of water balance through a feedback system. The osmoreceptors of the hypothalamus respond to an increase in the osmolarity of the blood plasma, as a result of which the secretion of vasopressin by the posterior pituitary gland is stimulated, and thirst also arises. The kidneys respond via receptors to increased levels of vasopressin by increasing water reabsorption, resulting in a decrease in plasma osmolarity due to its dilution with water.[121]
Variation in the rate of water excretion is an important survival function for mammals that have limited access to water.[119] The most prominent feature of the mammalian kidneys are the loops of Henle, they are the most efficient way to reabsorb water and create concentrated urine, which allows you to save water in the body.[122] After passing through the loop of Henle, the fluid becomes hypertonic relative to the blood plasma.[123] Mammalian kidneys combine nephrons with short and long loops of Henle.[124] The ability to concentrate urine is determined mainly by the structure of the medulla and the length of the loops of Henle.[125]
Endocrine function
In addition to excretory, the kidneys also perform an endocrine function, they produce certain hormones. The juxtaglomerular cells of the kidneys produce renin, which is a key regulator of the renin–angiotensin system, which is responsible for blood pressure regulation.[24]
The production of erythropoietin by the kidneys is responsible for the differentiation of erythroid progenitor cells in the bone marrow into erythrocytes and is induced by hypoxia. Thus, with a lack of oxygen, the number of red blood cells in the blood increases, and they are responsible for transporting oxygen.[25]
The kidneys are involved in the metabolism of vitamin D. In the liver, vitamin D is converted to calcifediol (25OHD), while the kidneys convert calcifediol to calcitriol (1,25(OH)2D), which is the active form of the vitamin and is essentially a hormone. Vitamin D is involved in the formation of bones and cartilage, and also performs a number of other functions, for example, it is involved in the functioning of the immune system.[26]
Blood pressure regulation
Some mammalian internal organs, including the kidneys and lung, are designed to function within normal blood pressure levels and normal blood volume levels, and blood pressure itself is also affected by changes in blood volume levels. Therefore, maintaining a constant blood volume for mammals is a very important function of the body.[126] The stable level of blood volume is influenced by the glomerular filtration rate, the function of individual parts of the nephron, the sympathetic nervous system and the renin-angiotensin-aldosterone system.[127]
In the walls of the afferent arterioles at the entrance to the renal glomeruli, there are juxtaglomerular cells. These cells are sensitive to changes in the minute volume of blood circulation, and to the composition and volume of the extracellular fluid, producing renin in response to changes in their level.[128] Once in the bloodstream, renin converts angiotensinogen to angiotensin I. Angiotensin I is further cleaved by the angiotensin-converting enzyme to angiotensin II, which is a potent vasoconstrictor that increases blood pressure.[128] In addition to angiotensin II, other biologically active substances can be formed in mammals. Angiotensin II can be cleaved to angiotensin III, angiotensin IV and angiotensin (1–7).[129]
Acid-base balance
Maintaining acid-base balance is vital because changes in pH levels affect virtually every biological process in the body.[130] In a typical mammal, a normal average pH level is around 7.4.[131] As in the case of other vertebrates in mammals, the acid-base balance is maintained mainly by the bicarbonate buffer system (HCO3-/CO2), which allows maintaining a constant pH level of the blood and extracellular fluid.[132] This buffer system is described by the following equation:[133]
The regulation of the acid-base balance through the bicarbonate buffer system is provided by the lungs and kidneys.[132] The lungs regulate CO2 (carbon dioxide) level, while the kidneys regulate HCO3- and H+ (bicarbonate and hydrogen ions).[133] The kidneys play a key role in maintaining a constant level of acid-base balance in mammals.[21] In the glomeruli, HCO3- is completely filtered into primary urine.[133] To maintain a constant pH, the kidneys reabsorb almost all of the HCO3- from primary urine back into the bloodstream and secrete H+ into the urine, oxidizing the urine.[134]
Reabsorption of HCO3- occurs in the proximal tubule, in the ascending limb of the loop of Henle, and to a lesser extent in the distal convoluted tubule of the nephron. H+ secretion is carried out mainly through Na+/H+ exchangers in the tubules of the nephron.[134] The collecting ducts are involved in the energy-dependent secretion of H+.[135] When H+ ions enter the urine, they can combine with filtered HCO3- to form carbonic acid H2CO3, which is being converted into CO2 and H2O (water) by the luminal carbonic anhydrase. The formed CO2 diffuses into the cells of the tubules, where it combines with H2O with the help of cytosolic carbonic anhydrase and forms HCO3-, which then returns to the bloodstream, and the formed H+ ion is secreted into the urine. Some of the H+ ions are secreted at an energy cost through an ATP-dependent mechanism.[134]
The excreted urine is slightly acidic. The excretion of H+ together with urine also occurs through buffer systems, in particular, NH4+ (ammonium).[136] Only a small amount of NH4+ is filtered through the glomerulus; [136] most of the ammonium excreted is the result of H+ ion oxidation of NH3 (ammonia) formed in the cells of the proximal convoluted tubule, which is secreted into the lumen of the tubule either as NH3 or as NH4+.[137] The formation of ammonia is also accompanied by the formation of new HCO3-, which replenishes the extracellular buffer system.[137] In the thick ascending tubule of the loop of Henle, on the contrary, NH4+ is absorbed, which causes its accumulation in the interstitium.[138] The final stage of urine oxidation occurs in the collecting ducts, where H+ ions are secreted with the involvement of ATP, and NH3 is transported from the interstitium and secreted into the urine, where NH3 is oxidized by H+ to form NH4+.[135] By regulating HCO3- reabsorption and H+ secretion, the kidneys help maintain blood pH homeostasis.[133]
Glucose homeostasis
Together with the liver,[139] the kidneys are involved in maintaining glucose homeostasis in the body of mammals.[22][140] The processes of filtration, reabsorption and consumption of glucose, as well as the production of glucose through gluconeogenesis, occur in the kidneys.[22][140] Glucose consumption (glycolysis) occurs primarily in the medulla, while gluconeogenesis occurs in the cortex. Hormonally, the process of gluconeogenesis in the kidneys is regulated by insulin and catecholamines.[22]
Evolution
Definitive mammalian kidney
The first mammals are believed to have appeared during the Permian period, which was characterized by cold nights in arid deserts and a strong seasonality with long, cold winters. It is likely that cold and aridity were significant factors of evolutionary pressure at that time. The development of warm-bloodedness in protomammals could lead to an increase in the intensity of blood circulation, and, accordingly, to an increase in blood pressure, which, in turn, increased the glomerular filtration rate of the kidneys. However, an increase in the glomerular filtration rate would also lead to an increase in the removal rate of water from the body.[141] All mammals have a thin segment of the tubule that is part of the loop of Henle. This segment is responsible for the concentration of urine and the reabsorption of water.[142] It can be assumed that the development of a water reabsorption mechanism could be part of the evolution of warm-bloodedness, rather than a direct adaptation to aridity.[141]
Adaptations to aridity
The ability to produce more concentrated urine is inversely dependent on the body mass of the mammals, that is, the smaller the mass of the animal, the more concentrated urine relative to animals with a larger mass its kidneys could produce during adaptation to an arid environment.[143] Some desert animals have evolved greater ability to concentrate urine than other animals.[144] The most concentrated urine among the studied species is produced by the Australian hopping mouse Notomys alexis,[143] whose kidneys have longer loops of Henley and an elongated renal papilla compared to the kidneys of other mammals.[145] The longer loops of Henley in the Australian hopping mouse make it possible to produce very concentrated urine[122] and survive in conditions of water scarcity.[143]
Adaptations to body mass
The simplest type of kidney in mammals is the unipapillary kidney, consisting of a cortex, medulla, and renal pelvis.[146] But the unipapillary kidney is limited by the number of nephrons at which it functions optimally.[13] It is assumed that unipapillary kidney was the original kidney structure in mammals, from which multilobar kidneys.[12]
More complex multilobar kidneys likely emerged as an adaptation to the increased body mass of mammals and the corresponding need for an increase in the number of nephrons in the kidneys.[13] A further adaptation mechanism is an increase in the size of the renal glomeruli in large mammals (and, accordingly, an increase in the length of the tubules), as in elephants, in which the diameter of the glomerulus can be 2 times larger than in killer whales.[78]
The appearance of reniculate kidneys was probably the result of adaptation to both an increase in body mass and habitats.[12][13] Reniculate kidneys probably allow the number of nephrons to be increased by adding renculi without the need to increase tubule length as the organ size increases.[78]
Reniculate kidneys
Reniculate kidneys are typical mainly for marine mammals. They are believed to be an adaptation both to the large body mass, allowing the number of nephrons to increase by increasing the number of renculi, and to a diet with large amounts of saline water, as well as an adaptation for long term diving. Consumption of excess salt leads to intracellular dehydration, resulting in a need for rapid removal of excess salt from the body, which in the case of reniculate kidneys is facilitated by an increase in the total surface area between the cortex and medulla.[12] The need to dive for long periods of time requires a reduction in the body's oxygen consumption,[147] while the kidneys are an energy-consuming organ,[148] so the glomerular filtration rate decreases during diving.[147] In contrast, the glomerular filtration rate is very high between dives.[12]
Development
Stages of kidney development
In mammals, the final kidney is the metanephric kidney, but kidneys development occurs in three stages with the development of three different types of kidney during embryonic period: pronephros, mesonephros and metanephros.[149][150] All three types develop from the intermediate mesoderm sequentially in the cranio-caudal direction (in the direction from the side of the head to the tail of the body).[151][150] First, the pronephros is formed, in mammals it is considered rudimentary, that is, it does not function.[149] Then, caudal to the pronephros, the mesonephros develops, which is the functioning kidney of the embryo.[149][150] Subsequently, the mesonephros degrades in females, and in males it participates in the development of the reproductive system. The third stage is the formation of a metanephros in the caudal part of the embryo, which is a permanent kidney.[149]
Metanephros development

The metanephros develops from the ureteric bud, which is an outgrowth on the caudal part of the nephric duct,[153][154] and the metanephrogenic blastema, which is part of the intermediate mesoderm surrounding the ureteral bud.[155][156] The development of metanephros begins with the induction of a metanephrogenic blastema by the ureteric bud.[156][149] While the kidney develops, the metanephrogenic blastema and ureteric bud reciprocally induce each other.[149] Growing into the mesoderm, the ureteric bud branches and transforms into a tree structure that will eventually become the ureter, renal pelvis, major and minor calyces, renal papillae, and collecting ducts.[157] At the same time, at the tips of the collecting ducts, the mesoderm differentiates into epithelial cells that form nephron tubules[158] (processes of epithelialization and tubulogenesis occur).[159] Vascular system of the kidney is also developed with the development of nephrons, with large vessels branching from the dorsal aorta.[159]
In some mammals, kidney organogenesis ends before birth, while in others it may continue for some time into the postpartum period[160] (for example, in rodents it ends about a week after birth).[161] When the formation of new nephrons (nephrogenesis) ends, the number of nephrons in the kidney becomes final.[160]
Postnatal maturation
After birth and in the postnatal period, the kidneys are functionally immature; functional development of the kidneys in all mammals lags behind the anatomical development.[162] In the postnatal period, the mass of the tubules is not large enough, so the ability to reabsorb fluids is reduced compared to the kidneys of adult mammals.[163] During this period, hypertrophy and hyperplasia of the tubules occurs, and the kidneys increase in size. The period during which fully functional kidneys form varies significantly between mammalian species. In rats, the kidneys quickly become fully functional, while in monkeys it takes 5 months.[162]
Injury and diseases
Kidney diseases or disorders may be congenital, inherited, non-infectious, and infectious.[29] Diseases vary between mammalian species. Some diseases may be specific only to some species, while the others may be more common in one species and less common in another.[32] For example, chronic progressive nephropathy is common in mice, rats and naked mole-rats,[164] but at the same time there is no analogous disease in humans.[165]
Congenital and inherited anomalies
Congenital anomalies and hereditary disorders of the kidneys among mammals are rare, but can have a significant impact on kidney function,[166] in some cases they can cause death in the early neonatal period.[167] Among the anomalies of kidney development are hypoplasia and dysplasia of the kidneys (dysplasia can be unilateral or bilateral), agenesis (absence) of one or both kidneys, polycystic kidney disease, simple renal cysts, perirenal pseudocysts, doubled or tripled renal arteries, malposition of the kidneys, horseshoe kidney and nephroblastoma.[167]
Non-infectious diseases
Non-infectious diseases of the kidney include acute kidney injury, chronic kidney disease,[168] glomerular diseases[169] and tubular diseases (renal tubular acidosis, Fanconi syndrome and renal glycosuria).[170] In small mammals, renal neoplasms are rare but usually are not benign.[171] Renal neoplasms and abscesses are rare in ruminants.[172] Obstructive uropathy (obstruction of urine flow from one or both kidneys) can lead to hydronephrosis with dilatation of the renal pelvis.[173] Kidney stones can also be formed in the kidneys (nephrolithiasis).[174]
The cause of acute kidney injury in most cases is ischemic or toxic injury. Mammalian kidneys are susceptible to ischemic injury because mammals lack a renal-portal system, and as a result, vascular vasoconstriction in the glomeruli can lead to decreased blood supply to the entire kidney. The kidneys are susceptible to toxic injury, since toxins are reabsorbed in the tubules along with most of the filtered substances.[35] The kidneys are able to restore their functionality after acute injury, but it also can progress into chronic kidney disease. Chronic kidney disease is characterised by loss of function of the kidney tissues, and the disease is usually progressive.[168]
Infectious diseases
Kidney infections in small mammals are usually caused by aerobic bacteria, including Escherichia coli, staphylococci, enterococci, and streptococci.[175] Fungal and parasitic infections of the kidney are rare in small mammals.[175] Pyelonephritis is usually caused by bacteria that enter the kidney through the ascending route from the lower parts of the urinary system, in rare cases through the blood (descending hematogenous route).[30] In ruminants, pyelonephritis is most often caused by the bacteria Corynebacterium renale and Escherichia coli.[31] Fish-eating mammals (such as minks and dogs) can become infected with the giant kidney worm Dioctophyme renale.[33] Pigs can become infected with the Stephanurus dentatus worm, which is found throughout the world, but is more common in the tropics and subtropics.[34][33] Kidney infections are considered rare among marine mammals.[176]
Ageing
After maturation, the kidneys slowly begin to undergo ageing processes, which are characterized by changes in anatomy, physiology, function and regenerative capabilities. During the life of mammals, glomerulosclerosis affects glomeruli, the basement membrane thickens, the tubules undergo atrophic changes, and the renal interstitium fibrosis increases. The number of functioning nephrons gradually decreases throughout the life. In terms of function, the glomerular filtration rate decreases and the ability to concentrate urine decreases, too. Age-related changes themselves may not be noticeable and may not lead to kidney failure or disease, but are a risk factor for kidney or urinary tract diseases.[42]
Repair and regeneration
Unlike more primitive vertebrates such as fish, in mammals nephrogenesis ends before or some time after birth,[38] caused by the loss of the condensed mesenchyme of the metanephrogenic blastema.[177] As a result, new nephrons cannot form in adults,[38] and after injuries, the kidneys of adult mammals cannot regenerate through the formation of new nephrons.[36] However, kidneys have other compensatory and regenerative mechanisms for restoring their function.[39]
Compensatory capabilities
In the case of unilateral nephrectomy, the load on the remaining kidney increases, increasing the rate of filtration and reabsorption and leading to changes in the nephrons themselves. The renal glomerulus may double or triple in diameter. These compensatory changes are similar to the changes in nephrons that occur after birth as the kidney grows.[178] Resection of kidney tissue also does not cause kidney regeneration,[179] however, compensatory changes can also occur after kidney damage if it leads to a significant decrease in the number of nephrons in the kidneys.[180]
Nephron regeneration
Within a single nephron, regenerative abilities differ between its parts.[181] In acute toxic and ischemic injuries, the tubules are able to regenerate and restore the function of the nephron.[178] In particular, the proximal part of the nephron, through which up to two-thirds of the primary urine is absorbed,[182] has the ability to regenerate.[183] This part of the nephron in mammals is most at risk of ischemic or toxic damage.[182] In addition, the repair of nephrons occurs in the course of normal physiological activity throughout the life due to the shedding of tubular epithelial cells.[184] The glomerulus has a complex structure, and its ability to recover after injury is limited.[185] Mesangial and endothelial cells are able to proliferate and restore their population after injury. On the contrary, podocytes do not proliferate under normal conditions.[186]
Healing after injury
If minor damage to the nephron tubules occurs, the lost cells are replaced by new ones, and the epithelium regenerates, restoring its structure and function. In moderate to severe injuries with large cell loss, the chances of regeneration of the tubular epithelium are reduced.[37] In such cases, damage leads to inflammatory and fibrotic responses, and regenerative tissue repair is impaired.[37] Such a reaction is typical for acute kidney injury.[41] Fibrosis is the second line of body defences,[187] which was supposed to reduce possible hemorrhage and fight possible infection during the evolution of mammals.[41] Renal fibrosis is the result of failed kidney healing and associated with renal dysfunction,[188] but it was suggested that it might support survival of non-injured and partially injured nephrons.[189] Chronic kidney injury is characterized by fibrosis, scarring, and loss of tissue function.[40]
References
- ↑ 1.0 1.1 Abdalla 2020, p. 1, Abstract.
- ↑ Withers, Cooper, Maloney et al. 2016, p. 25, 1.2.8 Excretion.
- ↑ 3.0 3.1 Keogh, Kilroy, Bhattacharjee 2020, p. 8, 7.3. Mammals.
- ↑ 4.0 4.1 4.2 4.3 Eurell, Frappier 2006, Kidney : General Organisation.
- ↑ 5.0 5.1 5.2 5.3 5.4 Withers, Cooper, Maloney et al. 2016, p. 250, 3.6.3 The Kidney.
- ↑ 6.0 6.1 Fenton, Knepper 2007, p. 679, Abstract.
- ↑ 7.0 7.1 , Wikidata Q117034134
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 WHO 1991, p. 49, 3.4 Species, strain, and sex differences in renal structure and function.
- ↑ 9.0 9.1 9.2 9.3 9.4 Breshears, Confer 2017, p. 617, Structure.
- ↑ 10.0 10.1 10.2 10.3 10.4 Ortiz 2001, p. 1832, Kidney structure.
- ↑ 11.0 11.1 Kriz, Kaissling 2012, p. 595, Renal vasculature.
- ↑ 12.0 12.1 12.2 12.3 12.4 Zhou, Rong, Guo et al. 2023, p. 2, Introduction.
- ↑ 13.0 13.1 13.2 13.3 Zhou, Rong, Guo et al. 2023, p. 6, The Evolution of Renal Structures Was Driven by Body Size and Habitats in Mammals.
- ↑ 14.0 14.1 Davidson 2009, Figure 1. Structure of the mammalian kidney.
- ↑ Dantzler 2016.
- ↑ Casotti, Lindberg, Braun 2000, p. R1723.
- ↑ Casotti, Lindberg, Braun 2000, p. R1722-R1723.
- ↑ Little, McMahon 2012, p. 1, Summary.
- ↑ Little, McMahon 2012, p. 2, An Overview of Cell Players and Cellular Processes in Metanephric Kidney Development.
- ↑ , Wikidata Q30419294
- ↑ 21.0 21.1 , Wikidata Q28972309
- ↑ 22.0 22.1 22.2 22.3 , Wikidata Q57012122
- ↑ , https://books.google.com/books?id=Ugq5BgAAQBAJ&pg=PA198, Wikidata Q117041834
- ↑ 24.0 24.1 , Wikidata Q30431545
- ↑ 25.0 25.1 , Wikidata Q89620015
- ↑ 26.0 26.1 , Wikidata Q33783519
- ↑ , https://books.google.com/books?id=1zP4bYDWq_wC&pg=PA506, Wikidata Q117043359
- ↑ , https://books.google.com/books?id=5aZHEAAAQBAJ&pg=PA5, Wikidata Q117043609
- ↑ 29.0 29.1 "Urinary System" (in en). https://www.msdvetmanual.com/urinary-system.
- ↑ 30.0 30.1 "Pyelonephritis in Small Animals" (in en). https://www.merckvetmanual.com/urinary-system/infectious-diseases-of-the-urinary-system-in-small-animals/pyelonephritis-in-small-animals.
- ↑ 31.0 31.1 , Wikidata Q38959395
- ↑ 32.0 32.1 , pp. 231-232, Wikidata Q122870348
- ↑ 33.0 33.1 33.2 , p. 1532, https://books.google.com/books?id=Jpg1ysgVn-AC&pg=PA1532, Wikidata Q122851992
- ↑ 34.0 34.1 "Swine Kidney Worm Infection" (in en). https://www.merckvetmanual.com/urinary-system/infectious-diseases-of-the-urinary-system-in-large-animals/swine-kidney-worm-infection.
- ↑ 35.0 35.1 , Wikidata Q38248788
- ↑ 36.0 36.1 Little, McMahon 2012, p. 12, 7 Reassessing Renal Disease, Repair, and Regeneration Using Developmental Biology.
- ↑ 37.0 37.1 37.2 Kumar 2018, p. 28, Figure 1 Schematic illustration highlighting patchy regenerative/reparative processes after mammalian acute kidney injury.
- ↑ 38.0 38.1 38.2 Davidson 2011, p. 1435, Introduction.
- ↑ 39.0 39.1 Davidson 2011, p. 1437-1439, Postnatal regenerative response of the mammalian kidney. Cite error: Invalid
<ref>tag; name "FOOTNOTEDavidson20111437-1439Postnatal regenerative response of the mammalian kidney" defined multiple times with different content - ↑ 40.0 40.1 H. Haller; S. Sorrentino, "Chapter 34 - Kidney", pp. 811, https://books.google.com/books?id=Xc1SHo0EHfYC&pg=PA811, Wikidata Q118139734
- ↑ 41.0 41.1 41.2 41.3 41.4 , Wikidata Q43573882
- ↑ 42.0 42.1 Breshears, Confer 2017, p. 637, Aging of the Kidney.
- ↑ , Wikidata Q117050923
- ↑ , https://books.google.com/books?id=BR8KAAAAQBAJ&pg=PA569, Wikidata Q117048829
- ↑ , Wikidata Q117048961
- ↑ 46.0 46.1 46.2 , https://books.google.com/books?id=qpQ9y-vXovoC&pg=PA319, Wikidata Q117048995
- ↑ De Martino, Cesare; Allen, Delmas; Accinni, Lidia, "Microscopic structure of the kidney", pp. 53–82, https://doi.org/10.1007/978-1-4613-2575-8_4, Wikidata Q117050154
- ↑ 48.0 48.1 Davidson 2009, Figure 1 Structure of the mammalian kidney.
- ↑ , pp. 16-18, Wikidata Q117051109
- ↑ 50.0 50.1 50.2 Mammalian kidney at the Encyclopædia Britannica
- ↑ "Excretion - Mammals" (in en). https://www.britannica.com/science/excretion/Mammals.
- ↑ Kriz, Kaissling 2012, p. 602, Figure 20.9 Schematic of nephrons and collecting duct.
- ↑ "Anatomy of the Kidney & Ureter". U. S. National Cancer Institute. https://training.seer.cancer.gov/kidney/anatomy/.
- ↑ , https://books.google.com/books?id=Hb1BXjgb0McC&pg=PA177, Wikidata Q117066071
- ↑ Davidson 2009, p. 2, Figure 1. Structure of the mammalian kidney.
- ↑ , Wikidata Q28388052
- ↑ Grant Maxie 2015, p. 379, Anatomy.
- ↑ 58.0 58.1 Sands, Layton 2012, p. 1464, Kidney Structure.
- ↑ Sands, Verlander 2004, p. 4-5, Figure 1.1 A long-looped and short-looped nephron together with the collecting system.
- ↑ Kriz, Kaissling 2012, p. 600, Nephrons and Collecting Duct System.
- ↑ 61.0 61.1 61.2 Kriz, Kaissling 2012, p. 595, Kidney Types and Renal Pelvis.
- ↑ 62.0 62.1 Sands, Verlander 2004, p. 6, Cortex.
- ↑ 63.0 63.1 , pp. 19, https://books.google.com/books?id=25_cDQAAQBAJ&pg=PA19, Wikidata Q117066851
- ↑ Grant Maxie 2015, p. 378, Anatomy.
- ↑ Davidson 2009, Overview of kidney structure and embryonic development.
- ↑ 66.0 66.1 , pp. 357, https://books.google.com/books?id=dW6eBQAAQBAJ&pg=PA357, Wikidata Q117068124
- ↑ 67.0 67.1 Sands, Verlander 2004, p. 8, Outer Medulla.
- ↑ W. Kriz, "Structure and Function of the Renal Medulla", pp. 3–10, doi:10.1007/978-3-642-69863-7_1, https://link.springer.com/chapter/10.1007/978-3-642-69863-7_1, Wikidata Q117072065
- ↑ Keogh, Kilroy, Bhattacharjee 2020, p. 9, 7.3.1. Mammalian kidneys: overall morphology.
- ↑ 70.0 70.1 70.2 Casotti, Lindberg, Braun 2000, p. R1722.
- ↑ Dantzler 2016, p. 20, 2.2.6 Mammals.
- ↑ Abdalla 2020, p. 3, 3. Results and discussion.
- ↑ , Wikidata Q48636042
- ↑ , pp. 286, https://books.google.com/books?id=0rDhBwAAQBAJ&pg=PA286, Wikidata Q117074872
- ↑ Dantzler 2016, p. 19—20, 2.2.6 Mammals.
- ↑ , Wikidata Q54957107
- ↑ Keogh, Kilroy, Bhattacharjee 2020, p. 8, 7.3.1. Mammalian kidneys: overall morphology.
- ↑ 78.0 78.1 78.2 , Wikidata Q104492546
- ↑ Russell, Hong, Windsor et al. 2019, p. 6, Detailed Features of Human and Mammalian Renal Lymphatic Anatomy : Renal Interstitium.
- ↑ Kriz, Kaissling 2012, p. 602, Interstitium : Definition.
- ↑ 81.0 81.1 , Wikidata Q36123584
- ↑ 82.0 82.1 Russell, Hong, Windsor et al. 2019, p. 6, Detailed Features of Human and Mammalian Renal Lymphatic Anatomy : Morphology of Renal Lymph Vessels.
- ↑ Kriz, Kaissling 2012, p. 602, Interstitial Fibroblasts.
- ↑ Breshears, Confer 2017, p. 622, Interstitium.
- ↑ , Wikidata Q117085458
- ↑ , Wikidata Q34434949
- ↑ 87.0 87.1 , Wikidata Q93115184
- ↑ , Wikidata Q35589079
- ↑ Balzer, Rohacs, Susztak 2022, p. 1, Introduction.
- ↑ 90.0 90.1 90.2 90.3 Grant Maxie 2015, p. 379, Vascular supply.
- ↑ Kriz, Kaissling 2012, p. 596, Renal vasculature.
- ↑ , Wikidata Q117088499
- ↑ , pp. 782, https://books.google.com/books?id=m-eQUEUjG2UC&pg=PA782, Wikidata Q117088647
- ↑ Keogh, Kilroy, Bhattacharjee 2020, p. 6, 6. Renal portal system.
- ↑ , pp. 371, https://books.google.com/books?id=hcw2AAAAQBAJ&pg=PA371, Wikidata Q117089453
- ↑ , pp. 299, https://books.google.com/books?id=lKVqBgAAQBAJ&pg=PT299, Wikidata Q117089734
- ↑ , Wikidata Q42177011
- ↑ Russell, Hong, Windsor et al. 2019, p. 1, Introduction.
- ↑ , Wikidata Q100995658
- ↑ Russell, Hong, Windsor et al. 2019, p. 7, Renal Lymphatic Physiology under Normal Conditions : Formation of Renal Lymph.
- ↑ 101.0 101.1 , Wikidata Q84203623
- ↑ Russell, Hong, Windsor et al. 2019, p. 2, Anatomy of Renal Lymphatics : Renal Vascular Anatomy.
- ↑ 103.0 103.1 103.2 103.3 Russell, Hong, Windsor et al. 2019, p. 3, Comparative Renal Lymphatic Anatomy : Mammalian Renal Lymphatic Anatomy.
- ↑ Russell, Hong, Windsor et al. 2019, p. 9, Renal Lymphatic Physiology under Normal Conditions : Interstitial Fluid and Protein Drainage in the Medulla.
- ↑ 105.0 105.1 Russell, Hong, Windsor et al. 2019, p. 5, Detailed Features of Human and Mammalian Renal Lymphatic Anatomy : Medullary Lymphatics.
- ↑ Russell, Hong, Windsor et al. 2019, p. 5, Table 1. Comparison of renal lymphatic anatomy between species.
- ↑ 107.0 107.1 107.2 107.3 Kopp 2018, p. 6, 2.2 Intrarenal Distribution of Efferent Renal Sympathetic Nerves.
- ↑ , Wikidata Q101322955
- ↑ Kopp 2011, Abstract.
- ↑ Kriz, Kaissling 2012, p. 610, Nerves.
- ↑ Kopp 2011c, 7.1. Kidney.
- ↑ Kopp 2011b, 2.1. Neural Pathways.
- ↑ Kopp 2018, p. 1, Introduction.
- ↑ Kopp 2011, 8.1. Activation of Afferent Renal Sensory Nerves by Physiological Stimuli.
- ↑ 115.0 115.1 , Wikidata Q39762062
- ↑ , Wikidata Q28284934
- ↑ "Excretion - General features of excretory structures and functions". https://www.britannica.com/science/excretion/General-features-of-excretory-structures-and-functions.
- ↑ Bradley 2009, p. 121, 8.4 Terrestrial vertebrates.
- ↑ 119.0 119.1 119.2 119.3 119.4 Sands, Layton 2009, Introduction.
- ↑ , Wikidata Q57865766
- ↑ , Wikidata Q38399100
- ↑ 122.0 122.1 Schulte, Kunter, Moeller 2014, p. 718, Adapting to living on dry land: the water-retaining kidney was invented twice.
- ↑ "The loop of Henle, distal tubule and collecting duct", pp. 70–85, https://link.springer.com/chapter/10.1007/978-94-011-4086-7_6, retrieved 2023-03-14, Wikidata Q117104227
- ↑ , Wikidata Q43688352
- ↑ Abdalla 2020, p. 1—2, 1. Introduction.
- ↑ Bradley 2009, p. 164, 11.5 The mammalian kidney.
- ↑ , Wikidata Q28729911
- ↑ 128.0 128.1 Sequeira-Lopez, Maria Luisa S.; Gomez, R. Ariel (2021-04-02). "Renin Cells, the Kidney, and Hypertension" (in en). Circulation Research 128 (7): 887–907. doi:10.1161/CIRCRESAHA.121.318064. ISSN 0009-7330. PMID 33793334.
- ↑ , pp. 153, https://books.google.com/books?id=JeTwCAAAQBAJ&pg=PA153, Wikidata Q117104121
- ↑ Eladari 2014, p. 1623, Introduction.
- ↑ , pp. 637, https://books.google.com/books?id=BR8KAAAAQBAJ&pg=PA637, Wikidata Q117048829
- ↑ 132.0 132.1 , pp. 66, https://books.google.com/books?id=f5sACAAAQBAJ&pg=PA64, Wikidata Q117190520
- ↑ 133.0 133.1 133.2 133.3 James L. Lewis III (Jul 2021), "Acid-Base Regulation", MSD Manuals - Medical Professional Version, https://www.msdmanuals.com/professional/endocrine-and-metabolic-disorders/acid-base-regulation-and-disorders/acid-base-regulation, retrieved 2023-03-17
- ↑ 134.0 134.1 134.2 Eladari, Hasler, Féraille 2012, p. 84, Bicarbonate Absorption.
- ↑ 135.0 135.1 Eladari 2014, p. 1629, Renal ammonia handling : Fig. 3.
- ↑ 136.0 136.1 Eladari 2014, p. 1627, Renal ammonia handling.
- ↑ 137.0 137.1 Eladari 2014, p. 1627-1628, Renal ammonia handling.
- ↑ Eladari 2014, p. 1628, Renal ammonia handling : Fig.2.
- ↑ , pp. 534, https://books.google.com/books?id=4Ab8CAAAQBAJ&pg=PA534, Wikidata Q123236759
- ↑ 140.0 140.1 , pp. 77-78, https://books.google.com/books?id=Dw3vfMM3wiIC&pg=PA77, Wikidata Q123237143
- ↑ 141.0 141.1 Vize, Smith 2004, p. 352.
- ↑ Vize, Smith 2004, p. 351.
- ↑ 143.0 143.1 143.2 , Wikidata Q112795614
- ↑ , Wikidata Q121096094
- ↑ , Wikidata Q122965291
- ↑ Zhou, Rong, Guo et al. 2023, p. 1, Introduction.
- ↑ 147.0 147.1 Ortiz 2001, p. 1838, Apnea/simulated diving.
- ↑ , Wikidata Q33683963
- ↑ 149.0 149.1 149.2 149.3 149.4 149.5 Bush, Sakurai, Nigam 2012, p. 859, Overview.
- ↑ 150.0 150.1 150.2 Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Davidson 2009, 1. Overview of kidney structure and embryonic development.
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Bush, Sakurai, Nigam 2012, pp. 859-860, Overview.
- ↑ 156.0 156.1 Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Bush, Sakurai, Nigam 2012, p. 861-862, Development of the Metanephros.
- ↑ Bush, Sakurai, Nigam 2012, p. 860, Development of the Metanephros.
- ↑ 159.0 159.1 Bush, Sakurai, Nigam 2012, p. 861, Development of the Metanephros.
- ↑ 160.0 160.1 Bush, Sakurai, Nigam 2012, p. 882, Termination of the Kidney Development.
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ 162.0 162.1 Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ "Overview of Congenital and Inherited Anomalies of the Urinary System" (in en). https://www.msdvetmanual.com/urinary-system/congenital-and-inherited-anomalies-of-the-urinary-system/overview-of-congenital-and-inherited-anomalies-of-the-urinary-system.
- ↑ 167.0 167.1 "Renal Anomalies" (in en). https://www.merckvetmanual.com/urinary-system/congenital-and-inherited-anomalies-of-the-urinary-system/renal-anomalies.
- ↑ 168.0 168.1 "Renal Dysfunction in Small Animals" (in en). https://www.merckvetmanual.com/urinary-system/noninfectious-diseases-of-the-urinary-system-in-small-animals/renal-dysfunction-in-small-animals.
- ↑ "Glomerular Disease in Small Animals" (in en). https://www.merckvetmanual.com/urinary-system/noninfectious-diseases-of-the-urinary-system-in-small-animals/glomerular-disease-in-small-animals.
- ↑ "Renal Tubular Defects in Small Animals" (in en). https://www.merckvetmanual.com/urinary-system/noninfectious-diseases-of-the-urinary-system-in-small-animals/renal-tubular-defects-in-small-animals.
- ↑ "Neoplasia of the Urinary System in Small Animals" (in en). https://www.merckvetmanual.com/urinary-system/noninfectious-diseases-of-the-urinary-system-in-small-animals/neoplasia-of-the-urinary-system-in-small-animals.
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ "Obstructive Uropathy in Small Animals" (in en). https://www.merckvetmanual.com/urinary-system/noninfectious-diseases-of-the-urinary-system-in-small-animals/obstructive-uropathy-in-small-animals.
- ↑ "Urolithiasis in Small Animals" (in en). https://www.merckvetmanual.com/urinary-system/noninfectious-diseases-of-the-urinary-system-in-small-animals/urolithiasis-in-small-animals.
- ↑ 175.0 175.1 "Overview of Infectious Diseases of the Urinary System in Small Animals" (in en). https://www.merckvetmanual.com/urinary-system/infectious-diseases-of-the-urinary-system-in-small-animals/overview-of-infectious-diseases-of-the-urinary-system-in-small-animals.
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Davidson 2011, p. 1436, Cessation of mammalian nephrogenesis.
- ↑ 178.0 178.1 Davidson 2011, p. 1437, Postnatal regenerative response of the mammalian kidney.
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Davidson 2011, p. 1441, Conclusions and perspectives.
- ↑ Yang, Liu, Fogo 2014, What Is Kidney Regeneration?.
- ↑ 182.0 182.1 Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Davidson 2011, p. 1438, Postnatal regenerative response of the mammalian kidney.
- ↑ Yang, Liu, Fogo 2014, Introduction.
- ↑ Yang, Liu, Fogo 2014, Mechanisms of Kidney Regeneration.
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Nogueira, Pires, Oliveira 2017, p. 3, Impact of Renal Fibrosis on Human Health.
- ↑ Nogueira, Pires, Oliveira 2017, p. 2, Renal Fibrosis: Aetiology and Pathophysiology.
Lua error: Internal error: The interpreter has terminated with signal "24".
Bibliography
Books
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
Article in scientific journals
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
- Lua error: Internal error: The interpreter has terminated with signal "24".
External links
Lua error: Internal error: The interpreter has terminated with signal "24".
