Biology:NAD+ kinase
| NAD+ kinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
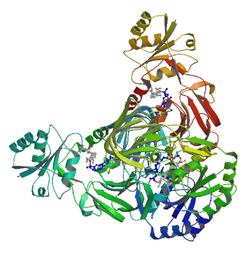 Ribbon diagram of NAD+ kinase in complex with substrates.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 2.7.1.23 | ||||||||
| CAS number | 9032-66-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
 Generic protein structure example |
NAD+ kinase (EC 2.7.1.23, NADK) is an enzyme that converts nicotinamide adenine dinucleotide (NAD+) into NADP+ through phosphorylating the NAD+ coenzyme.[2] NADP+ is an essential coenzyme that is reduced to NADPH primarily by the pentose phosphate pathway to provide reducing power in biosynthetic processes such as fatty acid biosynthesis and nucleotide synthesis.[3] The structure of the NADK from the archaean Archaeoglobus fulgidus has been determined.[1]
In humans, the genes NADK[4] and MNADK[5] encode NAD+ kinases localized in cytosol[4] and mitochondria,[5] respectively. Similarly, yeast have both cytosolic and mitochondrial isoforms, and the yeast mitochondrial isoform accepts both NAD+ and NADH as substrates for phosphorylation.[6][7]
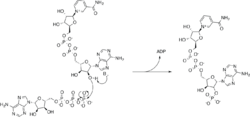
Reaction
ATP + NAD+ [math]\displaystyle{ \rightleftharpoons }[/math] ADP + NADP+
Mechanism
NADK phosphorylates NAD+ at the 2’ position of the ribose ring that carries the adenine moiety. It is highly selective for its substrates, NAD and ATP, and does not tolerate modifications either to the phosphoryl acceptor, NAD, or the pyridine moiety of the phosphoryl donor, ATP.[4] NADK also uses metal ions to coordinate the ATP in the active site. In vitro studies with various divalent metal ions have shown that zinc and manganese are preferred over magnesium, while copper and nickel are not accepted by the enzyme at all.[4] A proposed mechanism involves the 2' alcohol oxygen acting as a nucleophile to attack the gamma-phosphoryl of ATP, releasing ADP.
Regulation
NADK is highly regulated by the redox state of the cell. Whereas NAD is predominantly found in its oxidized state NAD+, the phosphorylated NADP is largely present in its reduced form, as NADPH.[8][9] Thus, NADK can modulate responses to oxidative stress by controlling NADP synthesis. Bacterial NADK is shown to be inhibited allosterically by both NADPH and NADH.[10] NADK is also reportedly stimulated by calcium/calmodulin binding in certain cell types, such as neutrophils.[11] NAD kinases in plants and sea urchin eggs have also been found to bind calmodulin.[12][13]
Clinical significance
Due to the essential role of NADPH in lipid and DNA biosynthesis and the hyperproliferative nature of most cancers, NADK is an attractive target for cancer therapy. Furthermore, NADPH is required for the antioxidant activities of thioredoxin reductase and glutaredoxin.[14][15] Thionicotinamide and other nicotinamide analogs are potential inhibitors of NADK,[16] and studies show that treatment of colon cancer cells with thionicotinamide suppresses the cytosolic NADPH pool to increase oxidative stress and synergizes with chemotherapy.[17]
While the role of NADK in increasing the NADPH pool appears to offer protection against apoptosis, there are also cases where NADK activity appears to potentiate cell death. Genetic studies done in human haploid cell lines indicate that knocking out NADK may protect from certain non-apoptotic stimuli.[18]
See also
References
- ↑ 1.0 1.1 PDB: 1SUW; "Crystal structures of an NAD kinase from Archaeoglobus fulgidus in complex with ATP, NAD, or NADP". Journal of Molecular Biology 354 (2): 289–303. Nov 2005. doi:10.1016/j.jmb.2005.09.026. PMID 16242716. https://zenodo.org/record/1259129.
- ↑ "Structural and functional properties of NAD kinase, a key enzyme in NADP biosynthesis". Mini Reviews in Medicinal Chemistry 6 (7): 739–46. Jul 2006. doi:10.2174/138955706777698688. PMID 16842123.
- ↑ "The power to reduce: pyridine nucleotides--small molecules with a multitude of functions". The Biochemical Journal 402 (2): 205–18. Mar 2007. doi:10.1042/BJ20061638. PMID 17295611.
- ↑ 4.0 4.1 4.2 4.3 "Structural and functional characterization of human NAD kinase". Biochemical and Biophysical Research Communications 288 (1): 69–74. Oct 2001. doi:10.1006/bbrc.2001.5735. PMID 11594753.
- ↑ 5.0 5.1 "MNADK, a Long-Awaited Human Mitochondrion-Localized NAD Kinase". Journal of Cellular Physiology 230 (8): 1697–701. Aug 2015. doi:10.1002/jcp.24926. PMID 25641397.
- ↑ "Characterization of NADH kinase from Saccharomyces cerevisiae". Journal of Biochemistry 105 (4): 588–93. Apr 1989. doi:10.1093/oxfordjournals.jbchem.a122709. PMID 2547755.
- ↑ "Localization of the NADH kinase in the inner membrane of yeast mitochondria". Journal of Biochemistry 105 (6): 916–21. Jun 1989. doi:10.1093/oxfordjournals.jbchem.a122779. PMID 2549021.
- ↑ "The measurement of triphosphopyridine nucleotide and reduced triphosphopyridine nucleotide and the role of hemoglobin in producing erroneous triphosphopyridine nucleotide values". The Journal of Biological Chemistry 242 (19): 4546–54. Oct 1967. doi:10.1016/S0021-9258(18)99573-6. PMID 4383634.
- ↑ "The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver". The Biochemical Journal 115 (4): 609–19. Dec 1969. doi:10.1042/bj1150609a. PMID 4391039.
- ↑ "Evidence that feedback inhibition of NAD kinase controls responses to oxidative stress". Proceedings of the National Academy of Sciences of the United States of America 103 (20): 7601–6. May 2006. doi:10.1073/pnas.0602494103. PMID 16682646. Bibcode: 2006PNAS..103.7601G.
- ↑ "Calmodulin-dependent NAD kinase of human neutrophils". Archives of Biochemistry and Biophysics 237 (1): 80–7. Feb 1985. doi:10.1016/0003-9861(85)90256-5. PMID 2982330.
- ↑ "Differential activation of NAD kinase by plant calmodulin isoforms. The critical role of domain I". The Journal of Biological Chemistry 272 (14): 9252–9. Apr 1997. doi:10.1074/jbc.272.14.9252. PMID 9083059.
- ↑ "Calmodulin activates NAD kinase of sea urchin eggs: an early event of fertilization". Cell 23 (2): 543–9. Feb 1981. doi:10.1016/0092-8674(81)90150-1. PMID 6258805.
- ↑ "The thioredoxin antioxidant system". Free Radical Biology & Medicine 66: 75–87. Jan 2014. doi:10.1016/j.freeradbiomed.2013.07.036. PMID 23899494.
- ↑ "Glutathione in cancer biology and therapy". Critical Reviews in Clinical Laboratory Sciences 43 (2): 143–81. 2006-01-01. doi:10.1080/10408360500523878. PMID 16517421.
- ↑ "Enhanced degradation of dihydrofolate reductase through inhibition of NAD kinase by nicotinamide analogs". Molecular Pharmacology 83 (2): 339–53. Feb 2013. doi:10.1124/mol.112.080218. PMID 23197646.
- ↑ "Suppression of Cytosolic NADPH Pool by Thionicotinamide Increases Oxidative Stress and Synergizes with Chemotherapy". Molecular Pharmacology 88 (4): 720–7. Oct 2015. doi:10.1124/mol.114.096727. PMID 26219913.
- ↑ "Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death". ACS Chemical Biology 10 (7): 1604–9. Jul 2015. doi:10.1021/acschembio.5b00245. PMID 25965523.
Further reading
- "A "double adaptor" method for improved shotgun library construction". Analytical Biochemistry 236 (1): 107–13. Apr 1996. doi:10.1006/abio.1996.0138. PMID 8619474.
- "Large-scale concatenation cDNA sequencing". Genome Research 7 (4): 353–8. Apr 1997. doi:10.1101/gr.7.4.353. PMID 9110174.
- "A human protein-protein interaction network: a resource for annotating the proteome". Cell 122 (6): 957–68. Sep 2005. doi:10.1016/j.cell.2005.08.029. PMID 16169070.
- "NAD kinase levels control the NADPH concentration in human cells". The Journal of Biological Chemistry 282 (46): 33562–71. Nov 2007. doi:10.1074/jbc.M704442200. PMID 17855339.
External links
- ENZYME entry on EC 2.7.1.23
- BRENDA entry on EC 2.7.1.23
- PDBe-KB provides an overview of all the structure information available in the PDB for Human NAD kinase
 |