Chemistry:Pregnenolone
| |
| |
| Names | |
|---|---|
| IUPAC name
3β-Hydroxypregn-5-en-20-one
| |
| Systematic IUPAC name
1-[(1S,3aS,3bS,7S,9aR,9bS,11aS)-7-Hydroxy-9a,11a-dimethyl-2,3,3a,3b,4,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-1-yl]ethan-1-one | |
| Other names
P5; 5-Pregnenolone; δ5-Pregnene-3β-ol-20-one; Pregn-5-en-3β-ol-20-one; NSC-1616
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H32O2 | |
| Molar mass | 316.485 g/mol |
| Melting point | 193 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
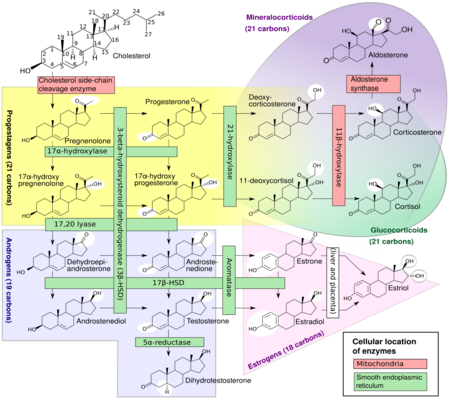
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids.[1] In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.[2]
In addition to its role as a natural hormone, pregnenolone has been used as a medication and supplement; for information on pregnenolone as a medication or supplement, see the pregnenolone (medication) article.
Biological function
Pregnenolone and its 3β-sulfate, pregnenolone sulfate, like DHEA, DHEA sulfate, and progesterone, belong to the group of neurosteroids that are found in high concentrations in certain areas of the brain, and are synthesized there. Neurosteroids affect synaptic functioning, are neuroprotective, and enhance myelinization. Pregnenolone and its sulfate ester may improve cognitive and memory function.[3] In addition, they may have protective effects against schizophrenia.[2]
Biological activity
Neurosteroid activity
Pregnenolone is an allosteric endocannabinoid, as it is a negative allosteric modulator of the CB1 receptor.[4][5] Pregnenolone is involved in a natural negative feedback loop against CB1 receptor activation in animals. It prevents CB1 receptor agonists like tetrahydrocannabinol, the main active constituent in cannabis, from fully activating the CB1.[6] A related compound AEF0117 has been derived from pregnenolone and is more specific for this type of activity.
Pregnenolone has been found to bind with high, nanomolar affinity to microtubule-associated protein 2 (MAP2) in the brain.[7][8] In contrast to pregnenolone, pregnenolone sulfate did not bind to microtubules.[7][8] However, progesterone did and with similar affinity to pregnenolone, although unlike pregnenolone, it did not increase binding of MAP2 to tubulin.[7][8] Pregnenolone was found to induce tubule polymerization in neuronal cultures and to increase neurite growth in PC12 cells treated with nerve growth factor.[7][8] As such, pregnenolone may control formation and stabilization of microtubules in neurons and may affect both neural development during prenatal development and neural plasticity during aging.[7][8]
Although pregnenolone itself does not possess these activities, its metabolite pregnenolone sulfate is a negative allosteric modulator of the GABAA receptor[9] as well as a positive allosteric modulator of the NMDA receptor.[10][11] In addition, pregnenolone sulfate has been shown to activate the transient receptor potential M3 (TRPM3) ion channel in hepatocytes and pancreatic islets causing calcium entry and subsequent insulin release.[12]
Nuclear receptor activity
Pregnenolone has been found to act as an agonist of the pregnane X receptor.[13]
Pregnenolone has no progestogenic, corticosteroid, estrogenic, androgenic, or antiandrogenic activity.[1]
Biochemistry
Biosynthesis
Pregnenolone is synthesized from cholesterol.[14] This conversion involves hydroxylation of the side chain at the C20 and C22 positions, with cleavage of the side chain.[14] The enzyme performing this task is cytochrome P450scc, located in the mitochondria, and controlled by anterior pituitary trophic hormones, such as adrenocorticotropic hormone, follicle-stimulating hormone, and luteinizing hormone, in the adrenal glands and gonads.[citation needed] There are two intermediates in the transformation of cholesterol into pregnenolone, 22R-hydroxycholesterol and 20α,22R-dihydroxycholesterol, and all three steps in the transformation are catalyzed by P450scc.[14] Pregnenolone is produced mainly in the adrenal glands, the gonads, and the brain.[4] Although pregnenolone is also produced in the gonads and brain, most circulating pregnenolone is derived from the adrenal cortex.[15]
To assay conversion of cholesterol to pregnenolone, radiolabeled cholesterol has been used.[16] Pregnenolone product can be separated from cholesterol substrate using Sephadex LH-20 minicolumns.[16]
Distribution
Pregnenolone is lipophilic and readily crosses the blood–brain barrier.[17] This is in contrast to pregnenolone sulfate, which does not cross the blood–brain barrier.[18][19]
Metabolism
Pregnenolone undergoes further steroid metabolism in one of several ways:
- Pregnenolone can be converted into progesterone. The critical enzyme step is two-fold using a 3β-hydroxysteroid dehydrogenase and a Δ5-4 isomerase. The latter transfers the double bond from C5 to C4 on the A ring. Progesterone is the entry into the Δ4 pathway, resulting in production of 17α-hydroxyprogesterone and androstenedione, precursor to testosterone and estrone. Aldosterone and corticosteroids are also derived from progesterone or its derivatives.
- Pregnenolone can be converted to 17α-hydroxypregnenolone by the enzyme 17α-hydroxylase (CYP17A1). Using this pathway, termed Δ5 pathway, the next step is conversion to dehydroepiandrosterone (DHEA) via 17,20-lyase (CYP17A1). DHEA is the precursor of androstenedione.
- Pregnenolone can be converted to androstadienol by 16-ene synthase (CYP17A1).
- Pregnenolone can be converted to pregnenolone sulfate by steroid sulfotransferase, and this conversion can be reversed by steroid sulfatase.
Levels
Normal circulating levels of pregnenolone are as follows:[15]
- Men: 10 to 200 ng/dL
- Women: 10 to 230 ng/dL
- Children: 10 to 48 ng/dL
- Adolescent boys: 10 to 50 ng/dL
- Adolescent girls: 15 to 84 ng/dL
Mean levels of pregnenolone have been found not to significantly differ in postmenopausal women and elderly men (40 and 39 ng/dL, respectively).[20]
Studies have found that pregnenolone levels are not significantly changed after surgical or medical castration in men, which is in accordance with the fact that pregnenolone is mainly derived from the adrenal glands.[21][22][23] Conversely, medical castration has been found to partially suppress pregnenolone levels in premenopausal women.[24][25] Similarly, an adrenalectomized premenopausal woman showed incompletely diminished circulating pregnenolone levels.[26]
Chemistry
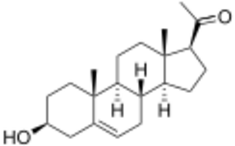
Pregnenolone is also known chemically as pregn-5-en-3β-ol-20-one.[27][28] Like other steroids, it consists of four interconnected cyclic hydrocarbons.[27][28] The compound contains ketone and hydroxyl functional groups, two methyl branches, and a double bond at C5, in the B cyclic hydrocarbon ring.[27][28] Like many steroid hormones, it is hydrophobic. The sulfated derivative, pregnenolone sulfate, is water-soluble.
3β-Dihydroprogesterone (pregn-4-en-3β-ol-20-one) is an isomer of pregnenolone in which the C5 double bond has been replaced with a C4 double bond.[1]
History
Pregnenolone was first synthesized by Adolf Butenandt and colleagues in 1934.[29]
References
- ↑ 1.0 1.1 1.2 "Pregnenolone". J. Clin. Endocrinol. Metab. 10 (4): 455–74. April 1950. doi:10.1210/jcem-10-4-455. PMID 15415436.
- ↑ 2.0 2.1 "Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence". Neuroscience 191: 78–90. September 2011. doi:10.1016/j.neuroscience.2011.06.076. PMID 21756978.
- ↑ "Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging". Brain Research. Brain Research Reviews 37 (1–3): 301–12. November 2001. doi:10.1016/S0165-0173(01)00135-7. PMID 11744095.
- ↑ 4.0 4.1 "Neurosteroids and potential therapeutics: Focus on pregnenolone". J. Steroid Biochem. Mol. Biol. 160: 78–87. 2016. doi:10.1016/j.jsbmb.2015.09.030. PMID 26433186.
- ↑ Pertwee, Roger G. (2015), "Endocannabinoids and Their Pharmacological Actions" (in en), Endocannabinoids, Handbook of Experimental Pharmacology, 231, Springer International Publishing, pp. 1–37, doi:10.1007/978-3-319-20825-1_1, ISBN 9783319208244, PMID 26408156
- ↑ Vallée, M., Vitiello, S., Bellocchio, L., Hébert-Chatelain, E., Monlezun, S., Martin-Garcia, E., Kasanetz, F., Baillie, GL., Panin, F., Cathala, A., Roullot-Lacarrière, V., Fabre, S., Hurst, DP., Lynch, DL., Shore, DM., Deroche-Gamonet, V., Spampinato, U., Revest, JM., Maldonado, R., Reggio, PH., Ross, RA., Marsicano, G., Piazza, PV. [1], "Pregnenolone Can Protect the Brain from Cannabis Intoxication", Science, January 2014 Science 343(6166):94-8.
- ↑ 7.0 7.1 7.2 7.3 7.4 "Neurosteroid regulation of central nervous system development". Pharmacol. Ther. 116 (1): 107–24. 2007. doi:10.1016/j.pharmthera.2007.04.011. PMID 17651807.
- ↑ 8.0 8.1 8.2 8.3 8.4 "Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor". Proc. Natl. Acad. Sci. U.S.A. 103 (12): 4711–6. 2006. doi:10.1073/pnas.0600113103. PMID 16537405. Bibcode: 2006PNAS..103.4711F.
- ↑ "Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons". Neuroscience Letters 90 (3): 279–84. August 1988. doi:10.1016/0304-3940(88)90202-9. PMID 3138576.
- ↑ "Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor". Molecular Pharmacology 40 (3): 333–6. September 1991. PMID 1654510. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=1654510.
- ↑ "Pregnenolone sulfate augments NMDA receptor mediated increases in intracellular Ca2+ in cultured rat hippocampal neurons". Neurosci Lett 141 (1): 30–4. July 1992. doi:10.1016/0304-3940(92)90327-4. PMID 1387199. https://zenodo.org/record/1258463.
- ↑ "Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells". Nature Cell Biology 10 (12): 1421–30. December 2008. doi:10.1038/ncb1801. PMID 18978782.
- ↑ "The PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signaling pathways". Recent Prog. Horm. Res. 54: 345–67; discussion 367–8. 1999. PMID 10548883.
- ↑ 14.0 14.1 14.2 Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 883–. ISBN 978-0-7817-6879-5. https://books.google.com/books?id=R0W1ErpsQpkC&pg=PA883.
- ↑ 15.0 15.1 "The Quest Diagnostics Manual. Endocrinology. Test Selection and Interpretation. Fourth Edition". p. 152. https://docplayer.net/86293048-The-quest-diagnostics-manual-endocrinology-test-selection-and-interpretation-fourth-edition.html.
- ↑ 16.0 16.1 "Pregnenolone separation from cholesterol using Sephadex LH-20 mini-columns". Journal of Chromatography A 190 (1): 256–262. 1980. doi:10.1016/S0021-9673(00)85545-4. https://zenodo.org/record/890904.
- ↑ "Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits". Biol. Psychiatry 73 (11): 1045–53. 2013. doi:10.1016/j.biopsych.2012.12.008. PMID 23348009.
- ↑ Jong Rho; Raman Sankar; Carl E. Stafstrom (18 June 2010). Epilepsy: Mechanisms, Models, and Translational Perspectives. CRC Press. pp. 479–. ISBN 978-1-4200-8560-0. https://books.google.com/books?id=8prMBQAAQBAJ&pg=PA479.
- ↑ CIBA Foundation Symposium (30 April 2008). Steroids and Neuronal Activity. John Wiley & Sons. pp. 101–. ISBN 978-0-470-51399-6. https://books.google.com/books?id=KeZlL3XbmQsC&pg=PA101.
- ↑ "Adrenocortical function in old age: response to acute adrenocorticotropin stimulation". J. Clin. Endocrinol. Metab. 54 (1): 187–91. January 1982. doi:10.1210/jcem-54-1-187. PMID 6274897.
- ↑ B.H. Vickery; J.J. Nestor; E.S. Hafez (6 December 2012). LHRH and Its Analogs: Contraceptive and Therapeutic Applications. Springer Science & Business Media. pp. 341–. ISBN 978-94-009-5588-2. https://books.google.com/books?id=am3yCAAAQBAJ&pg=PA341. "On the other hand there were no significant changes in serum levels of the steroid precursors pregnenolone and progesterone. [...] Unchanged levels of pregnenolone, in the presence of decreased 17α-OH-progesterone and testosterone concentrations, suggest that a block of 17-hydroxylase and 17,20-desmolase activity takes place in the human testis."
- ↑ "Inhibition of basal and adrenocorticotropin-stimulated plasma levels of adrenal androgens after treatment with an antiandrogen in castrated patients with prostatic cancer". J. Clin. Endocrinol. Metab. 59 (3): 422–6. September 1984. doi:10.1210/jcem-59-3-422. PMID 6086697.
- ↑ "Inhibition of serum androgen levels by chronic intranasal and subcutaneous administration of a potent luteinizing hormone-releasing hormone (LH-RH) agonist in adult men". Fertil. Steril. 37 (3): 416–24. March 1982. doi:10.1016/S0015-0282(16)46107-8. PMID 6800852.
- ↑ "Hormonal and biochemical changes during treatment of endometriosis with the luteinizing hormone-releasing hormone (LH-RH) agonist [D-Trp6,des-Gly-NH2(10)]LH-RH ethylamide". Fertil. Steril. 54 (2): 227–32. August 1990. doi:10.1016/S0015-0282(16)53694-2. PMID 2199228.
- ↑ "Clinical and hormonal effects of chronic gonadotropin-releasing hormone agonist treatment in polycystic ovarian disease". J. Clin. Endocrinol. Metab. 65 (4): 773–8. October 1987. doi:10.1210/jcem-65-4-773. PMID 3116031.
- ↑ "Serum steroid levels during the menstrual cycle in a bilaterally adrenalectomized woman". J. Clin. Endocrinol. Metab. 37 (4): 581–7. October 1973. doi:10.1210/jcem-37-4-581. PMID 4270264.
- ↑ 27.0 27.1 27.2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 665–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA665.
- ↑ 28.0 28.1 28.2 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 872–873. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA872.
- ↑ Butenandt, Adolf; Westphal, Ulrich; Cobler, Heinz (12 September 1934). "Über einen Abbau des Stigmasterins zu corpus-luteum-wirksamen Stoffen; ein Beitrag zur Konstitution des Corpusluteum-Hormons (Vorläuf. Mitteil.)". Berichte der Deutschen Chemischen Gesellschaft (A and B Series) 67 (9): 1611–1616. doi:10.1002/cber.19340670931.
 |