Chemistry:Butylated hydroxytoluene
| |
| |
| Names | |
|---|---|
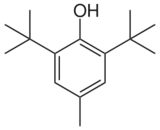
| Preferred IUPAC name
2,6-Di-tert-butyl-4-methylphenol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C15H24O | |
| Molar mass | 220.356 g/mol |
| Appearance | White to yellow powder |
| Odor | Slight, phenolic |
| Density | 1.048 g/cm3 |
| Melting point | 70 °C (158 °F; 343 K)[4] |
| Boiling point | 265 °C (509 °F; 538 K)[4] |
| 1.1 mg/L (20 °C)[1] | |
| log P | 5.32[2] |
| Vapor pressure | 0.01 mmHg (20 °C)[3] |
| Hazards | |
| Main hazards | Flammable |
| Safety data sheet | [5] |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H410 | |
| P273, P391, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 127 °C (261 °F; 400 K)[4] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
> 2,000 mg/kg (dermal, rat)[6] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
None[3] |
REL (Recommended)
|
TWA 10 mg/m3[3] |
IDLH (Immediate danger)
|
N.D.[3] |
| Related compounds | |
Related compounds
|
Butylated hydroxyanisole |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Butylated hydroxytoluene (BHT), also known as dibutylhydroxytoluene, is a lipophilic organic compound, chemically a derivative of phenol, that is useful for its antioxidant properties.[7] BHT is widely used to prevent free radical-mediated oxidation in fluids (e.g. fuels, oils) and other materials, and the regulations overseen by the U.S. F.D.A.—which considers BHT to be "generally recognized as safe"—allow small amounts to be added to foods. Despite this, and the earlier determination by the National Cancer Institute that BHT was noncarcinogenic in an animal model, societal concerns over its broad use have been expressed. BHT has also been postulated as an antiviral drug, but as of December 2022, use of BHT as a drug is not supported by the scientific literature and it has not been approved by any drug regulatory agency for use as an antiviral.[citation needed]
Natural occurrence
Phytoplankton, including the green algae Botryococcus braunii, as well as three different cyanobacteria (Cylindrospermopsis raciborskii, Microcystis aeruginosa and Oscillatoria sp.) are capable of producing BHT as a natural product.[8] The fruit lychee also produces BHT in its pericarp.[9] Several fungi (for example Aspergillus conicus) living in olives produce BHT.[10]
Production
Industrial production
The chemical synthesis of BHT in industry has involved the reaction of p-cresol (4-methylphenol) with isobutylene (2-methylpropene), catalyzed by sulfuric acid: [11]
- CH3(C6H4)OH + 2 CH2=C(CH3)2 → ((CH3)3C)2CH3C6H2OH
Alternatively, BHT has been prepared from 2,6-di-tert-butylphenol by hydroxymethylation or aminomethylation followed by hydrogenolysis.[citation needed]
Reactions
The species behaves as a synthetic analog of vitamin E, primarily acting as a terminating agent that suppresses autoxidation, a process whereby unsaturated (usually) organic compounds are attacked by atmospheric oxygen. BHT stops this autocatalytic reaction by converting peroxy radicals to hydroperoxides. It effects this function by donating a hydrogen atom:
- RO2• + ArOH → ROOH + ArO•
- RO2• + ArO• → nonradical products
where R is alkyl or aryl, and where ArOH is BHT or related phenolic antioxidants. Each BHT consumes two peroxy radicals.[12][non-primary source needed]
Applications
BHT is listed by the NIH Hazardous Substances Data Bank under several categories in catalogues and databases, such as food additive, household product ingredient, industrial additive, personal care product/cosmetic ingredient, pesticide ingredient, plastic/rubber ingredient and medical/veterinary/research.[13]
Food additive
BHT is primarily used as an antioxidant food additive.[14] In the United States, it is classified as generally recognized as safe (GRAS) based on a National Cancer Institute study from 1979 in rats and mice.[15][page needed] It is approved for use in the U.S. by the Food and Drug Administration: For example, 21 CFR § 137.350(a)(4) allows BHT up to 0.0033% by weight in "enriched rice",[16] while 9 CFR § 381.147](f)(1) allows up to 0.01% in poultry "by fat content".[17] It is permitted in the European Union under E321.[18]
BHT is used as a preservative ingredient in some foods. With this usage BHT maintains freshness or prevents spoilage; it may be used to decrease the rate at which the texture, color, or flavor of food changes.[19]
Some food companies have voluntarily eliminated BHT from their products or have announced that they were going to phase it out.[20]
Antioxidant
BHT is also used as an antioxidant in products such as metalworking fluids, cosmetics, pharmaceuticals, rubber, transformer oils, and embalming fluid.[21][22] In the petroleum industry, where BHT is known as the fuel additive AO-29, it is used in hydraulic fluids, turbine and gear oils, and jet fuels.[21][23][page needed] BHT is also used to prevent peroxide formation in organic ethers and other solvents and laboratory chemicals.[24] It is added to certain monomers as a polymerisation inhibitor to facilitate their safe storage.[25] Some additive products contain BHT as their primary ingredient, while others contain the chemical merely as a component of their formulation, sometimes alongside butylated hydroxyanisole (BHA).[26]
Cosmetics
The European Union restricts the use of BHT in mouthwash to .001% concentration, in toothpaste to .01% concentration, and to .8% in other cosmetics.[27]
Health effects
Like many closely related phenol antioxidants, BHT has low acute toxicity[6] (e.g., the desmethyl analog of BHT, 2,6-di-tert-butylphenol, has an -1">50 of >9 g/kg[11]). The US Food and Drug Administration classifies BHT as generally recognized as safe (GRAS) food preservative when used in an approved manner.[28][29] In 1979, the National Cancer Institute determined that BHT was noncarcinogenic in a mouse model.[15][needs update]
Nevertheless, the World Health Organization discussed a possible link between BHT and cancer risk in 1986,[30][page needed][verification needed][needs update] and some primary research studies in the 1970s–1990s reported both potential for increased risk and potential for decreased risk in the area of oncology.[31][32][33][non-primary source needed] Because of this uncertainty, the Center for Science in the Public Interest puts BHT in its "caution" column and recommends avoiding it.[34]
Based on various, disparate primary research reports, BHT has been suggested to have anti-viral activity,[35] and the reports divide into various study types. First, there are studies that describe virus inactivation—where treatment with the chemical results in disrupted or otherwise inactivated virus particles.[36][37][non-primary source needed] The action of BHT in these is akin to the action of many other organic compounds, e.g., quaternary ammonium compounds, phenolics, and detergents, which disrupt viruses by insertion of the chemical into the virus membrane, coat, or other structure,[38][39][40] which are established methods of viral disinfection secondary to methods of chemical oxidation and UV irradiation.[41][citation needed] In addition, there is a report of BHT use, topically against genital herpes lesions,[42][non-primary source needed] a report of inhibitory activity in vitro against pseudorabies (in cell culture),[43][non-primary source needed] and two studies, in veterinary contexts, of use of BHT to attempt to protect against virus exposure (pseudorabies in mouse and swine, and Newcastle in chickens).[43][44][non-primary source needed] The relevance of other reports, regarding influenza in mice, is not easily discerned.[45][46][non-primary source needed] Notably, this series of primary research reports does not support a general conclusion of independent confirmation of the original research results,[47] nor are there critical reviews appearing thereafter, in secondary sources, for the various host-virus systems studied with BHT.[48][49]
Hence, at present, the results do not present a scientific consensus in favour of the conclusion of the general antiviral potential of BHT when dosed in humans. Moreover, as of March 2020, no guidance from any of the internationally recognized associations of infectious disease specialists had advocated use of BHT products as an antiviral therapy or prophylactic.[50][51][52]
References
- ↑ KEMI Anställd [KEMI Staff] (1994). "Teknisk beskrivning av ämnet—2,6-Bis(tert-butyl)-4-metylfenol 1994 [Information on substances—2,6-Bis(tert-butyl)-4-methylphenol 1994"] (in sv,en). Sundyberg, SE: KEMI [Swedish Chemicals Agency]. http://apps.kemi.se/flodessok/floden/kemamne/BHT.htm. "Vattenlöslighet: 1,1 mg/L (20 °C) [Water solubility: 1.1 mg/L (20°C)]"
- ↑ "2,6-di-tert-butyl-4-methylphenol". https://www.chemsrc.com/en/cas/128-37-0_1067745.html.
- ↑ 3.0 3.1 3.2 3.3 NIOSH Pocket Guide to Chemical Hazards. "#0246". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0246.html.
- ↑ 4.0 4.1 4.2 "ICSC 0841 - BUTYLATED HYDROXYTOLUENE". March 1999. http://www.inchem.org/documents/icsc/icsc/eics0841.htm.
- ↑ "Safety data for 2,6-di-tert-butyl-p-cresol". http://ptcl.chem.ox.ac.uk/MSDS/DI/2,6-di-t-butyl-p-cresol.html.
- ↑ 6.0 6.1 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ "Understanding the Chemistry Behind the Antioxidant Activities of Butylated Hydroxytoluene (BHT): A Review". Eur. J. Med. Chem. 101: 295–312. 28 August 2015. doi:10.1016/j.ejmech.2015.06.026. PMID 26150290.
- ↑ "Production of Natural Butylated Hydroxytoluene as an Antioxidant by Freshwater Phytoplankton". Journal of Phycology 44 (6): 1447–1454. December 2008. doi:10.1111/j.1529-8817.2008.00596.x. PMID 27039859. http://ntur.lib.ntu.edu.tw/bitstream/246246/162863/1/22.pdf.
- ↑ Jiang, G; Lin, S; Wen, L; Jiang, Y; Zhao, M; Chen, F; Prasad, KN; Duan, X et al. (15 January 2013). "Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation.". Food Chemistry 136 (2): 563–8. doi:10.1016/j.foodchem.2012.08.089. PMID 23122098.
- ↑ Gharbi, Ines; Issaoui, Manel; El Gharbi, Sinda; Gazzeh, Nour‐Eddine; Tekeya, Meriem; Mechri, Beligh; Flamini, Guido; Hammami, Mohamed (2017). "Butylated hydroxytoluene (BHT) emitted by fungi naturally occurring in olives during their pre‐processing storage for improving olive oil stability". European Journal of Lipid Science and Technology 119 (11): 1600343. doi:10.1002/ejlt.201600343.
- ↑ 11.0 11.1 Helmut Fiege, Heinz-Werner Voges, Toshikazu Hamamoto, Sumio Umemura, Tadao Iwata, Hisaya Miki, Yasuhiro Fujita, Hans-Josef Buysch, Dorothea Garbe, Wilfried Paulus "Phenol Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a19_313 Article Online Posting Date: June 15, 2000.
- ↑ Burton G. W., Ingold K. U. (1981). "Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro". Journal of the American Chemical Society 103 (21): 6472–6477. doi:10.1021/ja00411a035.
- ↑ US Dept of Health & Human Services. Household Products Database. [1] .US EPA. InertFinder. [2]. US National Library of Medicine. Haz-Map. [3] . US National Library of Medicine. Hazardous Substances Data Bank. [4].
- ↑ "CFR - Code of Federal Regulations Title 21". https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.115.
- ↑ 15.0 15.1 Bioassay of Butylated Hydroxytoluene (BHT) for Possible Carcinogenicity, National Cancer Institute, CARCINOGENESIS Technical Report Series No. 150, 1979, 128 pp National Institutes of Health[page needed]
- ↑ "CFR - Code of Federal Regulations Title 21". https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=137.350.
- ↑ "9 CFR 3, Part 381.147 (Restrictions on the Use of Substances in Poultry Processing)". https://www.fda.gov/Food/FoodSafety/RetailFoodProtection/FoodCode/FoodCode2001/ucm092716.htm.
- ↑ "Scientific Opinion on the re-evaluation of butylated hydroxytoluene BHT (E 321) as a food additive | European Food Safety Authority". 7 March 2012. http://www.efsa.europa.eu/en/efsajournal/pub/2588.
- ↑ "Food Additives & Ingredients > Overview of Food Ingredients, Additives & Colors". 20 February 2020. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm094211.htm#types. "Types of Ingredients: Preservatives[.] What They Do: Prevent food spoilage from [...]; maintain freshness[.] Examples of Uses: Fruit sauces and jellies, beverages, baked goods, cured meats, oils and margarines, cereals, dressings, snack foods, fruits and vegetables[.] Names Found on Product Labels: Ascorbic acid, citric acid, sodium benzoate, calcium propionate, sodium erythorbate, sodium nitrite, calcium sorbate, potassium sorbate, BHA, BHT, EDTA, tocopherols (Vitamin E)[.]"
- ↑ Hamblin, James (11 February 2015). "The Food Babe: Enemy of Chemicals". The Atlantic. https://www.theatlantic.com/health/archive/2015/02/the-food-babe-enemy-of-chemicals/385301/. Retrieved 12 September 2015.
- ↑ 21.0 21.1 Yehye, Wageeh A.; Rahman, Noorsaadah Abdul; Ariffin, Azhar; Abd Hamid, Sharifah Bee; Alhadi, Abeer A.; Kadir, Farkaad A.; Yaeghoobi, Marzieh (2015-08-28). "Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): a review". European Journal of Medicinal Chemistry 101: 295–312. doi:10.1016/j.ejmech.2015.06.026. ISSN 1768-3254. PMID 26150290. https://pubmed.ncbi.nlm.nih.gov/26150290/.
- ↑ PubChem. "Butylated Hydroxytoluene" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/31404.
- ↑ Michael Ash, Irene Ash, Handbook of Preservatives, Synapse Information Resources, 2004. ISBN:1-890595-66-7.[page needed]
- ↑ "Solvents". https://www.sigmaaldrich.com/chemistry/solvents/learning-center/stabilizer-systems.html.
- ↑ Grohmann, Caio Vinícius Signorelli; Sinhoreti, Mário Alexandre Coelho; Soares, Eveline Freitas; Oliveira, Robson Ferraz de; Souza, Eduardo José de Carvalho; Geraldeli, Saulo (24 June 2022). "Effect of a polymerization inhibitor on the chemomechanical properties and consistency of experimental resin compo". Brazilian Dental Journal 33 (3): 92–98. doi:10.1590/0103-6440202204242. PMID 35766722.
- ↑ "BHA and BHT: A Case for Fresh?". Scientific American. 19 December 2013. https://www.scientificamerican.com/article/bha-and-bht-a-case-for-fresh/.
- ↑ Slavova, Siana (23 June 2023). "New restrictions for Butylated Hydroxytoluene (BHT) and Acid Yellow 3 apply as of July 2023" (in en). https://www.obelis.net/news/new-restrictions-bht-acid-yellow-3-cosmetic-products/.
- ↑ "SCOGS (Select Committee on GRAS Substances)". FDA.gov. https://www.accessdata.fda.gov/scripts/fdcc/?set=SCOGS.
- ↑ "CFR—Code of Federal Regulations Title 21". FDA.gov. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=172.115.
- ↑ Butylated hydroxytoluene (BHT) (Report). 40. World Health Organization: International Agency For Research On Cancer. 1986. pp. 161–206. https://monographs.iarc.fr/ENG/Monographs/vol1-42/mono40.pdf.[page needed]
- ↑ Kensler, TW; Egner, PA; Trush, MA; Bueding, E; Groopman, JD (1985). "Modification of aflatoxin B1 binding to DNA in vivo in rats fed phenolic antioxidants, ethoxyquin and a dithiothione". Carcinogenesis 6 (5): 759–763. doi:10.1093/carcin/6.5.759. PMID 3924431.
- ↑ Williams, GM; Iatropoulos, M. J (1996). "Inhibition of the hepatocarcinogenicity of aflatoxin B1 in rats by low levels of the phenolic antioxidants butylated hydroxyanisole and butylated hydroxytoluene". Cancer Letters 104 (1): 49–53. doi:10.1016/0304-3835(96)04228-0. PMID 8640745.
- ↑ Franklin, R. A (1976). "Butylated hydroxytoluene in sarcoma-prone dogs". Lancet 1 (7972): 1296. doi:10.1016/s0140-6736(76)91766-9. PMID 73719.
- ↑ "Two Preservatives to Avoid?". University of California Berkeley. February 1, 2011. http://www.berkeleywellness.com/healthy-eating/food-safety/article/two-preservatives-avoid.
- ↑ The term disparate here is purely descriptive, and not pejorative—each of the primary research reports that follow is distinct and dissimilar, and so they are as a set, disparate. Moreover, no group of articles constitute a series, reflecting long-term study of BHT in a host-virus pair by the same research team (the pair by Chetverikova et al. being the nearest to this).
- ↑ "Butylated Hydroxytoluene Inactivated Lipid-Containing Viruses". Science 188 (4183): 64–66. 4 April 1975. doi:10.1126/science.163494. PMID 163494. Bibcode: 1975Sci...188...64S.
- ↑ Kim, K. S; Moon, H. M; Sapienza, V; Carp, R. I; Pullarkat, R (1978). "Inactivation of cytomegalovirus and Semliki Forest virus by butylated hydroxytoluene". The Journal of Infectious Diseases 138 (1): 91–4. doi:10.1093/infdis/138.1.91. PMID 210237.
- ↑ Rutala, William A.; Weber, David J. (January 2015). "Disinfection, Sterilization, and Control of Hospital Waste". Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases: 3294–3309.e4. doi:10.1016/B978-1-4557-4801-3.00301-5. ISBN 9781455748013. https://www.sciencedirect.com/science/article/pii/B9781455748013003015. Retrieved 2020-03-13.
- ↑ Cook, Nigel; Knight, Angus; Richards, Gary P. (1 July 2016). "Persistence and Elimination of Human Norovirus in Food and on Food Contact Surfaces: A Critical Review". Journal of Food Protection 79 (7): 1273–94. doi:10.4315/0362-028X.JFP-15-570. PMID 27357051.
- ↑ Ferris Jabr (13 March 2020). "Why Soap Works - The New York Times". The New York Times. https://www.nytimes.com/2020/03/13/health/soap-coronavirus-handwashing-germs.html.
- ↑ "Persistence of Coronaviruses on Inanimate Surfaces and Their Inactivation With Biocidal Agents" (PDF). J. Hosp. Infect. 104 (3): 246–251. March 2020. doi:10.1016/j.jhin.2020.01.022. PMID 32035997. PMC 7132493. https://www.journalofhospitalinfection.com/article/S0195-6701(20)30046-3/pdf. Retrieved 14 March 2020.
- ↑ Richards, J. T; Katz, M. E; Kern, E. R (1985). "Topical butylated hydroxytoluene treatment of genital herpes simplex virus infections of guinea pigs". Antiviral Research 5 (5): 281–90. doi:10.1016/0166-3542(85)90042-7. PMID 2998276.
- ↑ 43.0 43.1 Pirtle, E. C; Sacks, J. M; Nachman, R. J (1986). "Antiviral effectiveness of butylated hydroxytoluene against pseudorabies (Aujeszky's disease) virus in cell culture, mice, and swine". American Journal of Veterinary Research 47 (9): 1892–5. PMID 3021025.
- ↑ Brugh, M (1977). "Butylated hydroxytoluene protects chickens exposed to Newcastle disease virus". Science 197 (4310): 1291–2. doi:10.1126/science.897670. PMID 897670. Bibcode: 1977Sci...197.1291B.
- ↑ Chetverikova, L. K; Ki'Ldivatov, I. Iu; Inozemtseva, L. I; Kramskaia, T. A; Filippov, V. K; Frolov, B. A (1989). "Factors of Antiviral Resistance in the Pathogenesis of Influenza in Mice" (in ru). Vestnik Akademii Meditsinskikh Nauk SSSR (11): 63–8. PMID 2623936.
- ↑ Chetverikova LK, Inozemtseva LI (1996). "Role of Lipid Peroxidation in the Pathogenesis of Influenza and Search for Antiviral Protective Agents" (in ru). Vestn Ross Akad Med Nauk 3 (3): 37–40. PMID 8672960.
- ↑ As of March 2020, there are no examples in this series presenting primary research that reproduces earlier reported results—the reports generally present research results on distinct host-virus systems, rather than follow-up studies on the same systems.
- ↑ Search of Pubmed in March 2020 with the main field search string, "(BHT OR butylated hydroxytoluene) AND antiviral [TIAB]", see next citation, to pull articles focused on antiviral effects of the agent produced a single review source, PMID 12122334, which is a review of the use of topical agents in treatment of herpes facialis and genitalis; this 18-year old review mentioning BHT in this topical application is irrelevant to its value as a general antiviral, and to its utility as an orally bioavailable agent in humans. See "Traitements locaux, antiviraux ou non, dans la prise en charge de l'herpès oro-facial et génital (grossesse et nouveau-né exclus)". Annales de Dermatologie et de Vénéréologie 129 (4–C2): 635–645. April 2002. PMID 12122334. https://www.em-consulte.com/en/article/153873. Retrieved 12 March 2020. "DOI, DERM-04-2002-129-4-C2-0151-9638-101019-ART18".
- ↑ "(BHT OR butylated hydroxytoluene) AND antiviral [TIAB - PubMed - NCBI"]. https://pubmed.ncbi.nlm.nih.gov/?term=(BHT+OR+butylated+hydroxytoluene)+AND+antiviral+%5BTIAB%5D.
- ↑ ISID Web Tools (12 March 2020). "You searched for BHT". ISID.org. International Society for Infectious Diseases (ISID). https://isid.org/search/BHT/. "There are 0 results for 'BHT'"
- ↑ "ESCMID—Search—Website Search—Search in Category". https://www.escmid.org/search/. Search for "bht".
- ↑ See for instance, this and the following two references: IDSA Web Tools (12 March 2020). "Search Results". IDSociety.org. Infectious Diseases Society of America (IDSA). https://www.idsociety.org/search-results?query=BHT#/score/DESC/0/BHT/. "No results found"
External links
 |


