Chemistry:6-Hydroxymelatonin
From HandWiki
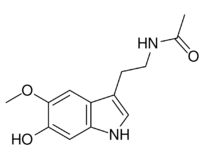
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-[2-(6-Hydroxy-5-methoxy-1H-indol-3-yl)ethyl]acetamide | |
| Other names
6-Oxymelatonin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H16N2O3 | |
| Molar mass | 248.282 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
6-Hydroxymelatonin (6-OHM) is a naturally occurring, endogenous, major active metabolite of melatonin.[1] Similar to melatonin, 6-OHM is a full agonist of the MT1 and MT2 receptors.[2][3] It is also an antioxidant and neuroprotective, and is even more potent in this regard relative to melatonin.[4][5]
See also
- N-Acetylserotonin (normelatonin)
- 5-Methoxytryptamine
References
- ↑ "Melatonin metabolism in the central nervous system". Curr Neuropharmacol 8 (3): 168–81. 2010. doi:10.2174/157015910792246164. PMID 21358968.
- ↑ "Pharmacology and function of melatonin receptors". FASEB J. 2 (12): 2765–73. 1988. doi:10.1096/fasebj.2.12.2842214. PMID 2842214.
- ↑ Browning, Christopher; Beresford, Isabel; Fraser, Neil; Giles, Heather (2000). "Pharmacological characterization of human recombinant melatonin mt1and MT2receptors". British Journal of Pharmacology 129 (5): 877–886. doi:10.1038/sj.bjp.0703130. ISSN 0007-1188. PMID 10696085.
- ↑ "Melatonin: new places in therapy". Biosci. Rep. 27 (6): 299–320. 2007. doi:10.1007/s10540-007-9052-1. PMID 17828452.
- ↑ "N-Acetylserotonin and 6-Hydroxymelatonin against Oxidative Stress: Implications for the Overall Protection Exerted by Melatonin". J Phys Chem B 119 (27): 8535–43. 2015. doi:10.1021/acs.jpcb.5b04920. PMID 26079042.
 |

