Chemistry:Denintuzumab mafodotin
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | CD19 |
| Clinical data | |
| Other names | SGN-19A |
| ATC code |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| KEGG | |
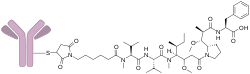
Denintuzumab mafodotin (INN; development codes SGN-19A or SGN-CD19A) is a humanized monoclonal antibody-drug conjugate designed for the treatment of CD19-positive acute lymphoblastic leukemia and B-cell non-Hodgkin lymphoma.[1][2][3][4] It consists of an anti-CD19 mAb linked to monomethyl auristatin F (MMAF), a cytotoxic agent.[5] This drug was developed by Seattle Genetics.
Denintuzumab refers to the anti-CD19 antibody, and mafodotin refers to MMAF and the chemical linkage.[6]
Clinical trials
The drug is in phase I clinical trials.[7] Preliminary phase I results for B-cell malignancies, including diffuse large B-cell lymphoma (DLBCL) and B-lineage acute lymphocytic leukemia (B-ALL) were presented at the ASH medical conference Dec 2015.[4]
Phase 2
A separate randomized phase 2 trial started in 2015 to evaluate SGN-CD19A in combination with R-ICE chemotherapy for second-line DLBCL.[4] A phase 2 clinical trial in front-line DLBCL is started in 2016.[4] Both trials were terminated by the sponsor based on portfolio prioritization.[8][9]
References
- ↑ "About Denintuzumab Mafodotin (SGN-CD19A; 19A)". Seattle Genetics. http://www.seattlegenetics.com/sgn-cd19a.
- ↑ Statement On A Nonproprietary Name Adopted By The USAN Council - Denintuzumab Mafodotin, American Medical Association.
- ↑ World Health Organization (2014). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 111". WHO Drug Information 28 (2). https://www.who.int/medicines/publications/druginformation/innlists/PL111.pdf.
- ↑ 4.0 4.1 4.2 4.3 "Seattle Genetics Highlights Data from Denintuzumab Mafodotin (SGN-CD19A) Antibody-Drug Conjugate Program at ASH 2015". December 6, 2015. https://www.businesswire.com/news/home/20151206005047/en/Seattle-Genetics-Highlights-Data-Denintuzumab-Mafodotin-SGN-CD19A.
- ↑ "Seattle Genetics Presents Data from Novel Antibody-Drug Conjugate (ADC) SGN-CD19A at ASH 2013". Seattle Genetics. https://finance.yahoo.com/news/seattle-genetics-presents-data-novel-150000223.html.
- ↑ "Statement on a nonproprietary name adopted by the USAN Council: Mafodotin". http://www.ama-assn.org/resources/doc/usan/mafodotin.pdf.
- ↑ "Search of: SGN-CD19A - List Results - ClinicalTrials.gov". https://clinicaltrials.gov/search/intervention=SGN-CD19A.
- ↑ Clinical trial number NCT02592876 for "Treatment Study of Denintuzumab Mafodotin (SGN-CD19A) Plus RICE Versus RICE Alone for Diffuse Large B-Cell Lymphoma" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02855359 for "Denintuzumab Mafodotin (SGN-CD19A) Combined With RCHOP or RCHP Versus RCHOP Alone in Diffuse Large B-Cell Lymphoma or Follicular Lymphoma" at ClinicalTrials.gov
 |
