Chemistry:Nivolumab
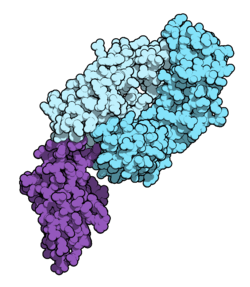 Fab fragment of nivolumab (blue) binding the extracellular domain of PD-1 (purple). From PDB entry 5ggr. | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | PD-1 |
| Clinical data | |
| Trade names | Opdivo |
| Other names | ONO-4538, BMS-936558, MDX1106 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614056 |
| License data | |
| Pregnancy category | |
| Routes of administration | Intravenous |
| Drug class | Immunotherapy[3] |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C6362H9862N1712O1995S42 |
| Molar mass | 143599.39 g·mol−1 |
Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer.[2] This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer.[6][7][2][8][9] It is administered intravenously.[6][7][2]
The most common side effects include fatigue, rash, musculoskeletal pain, pruritus (itching), diarrhea, nausea, asthenia (weakness), cough, dyspnea (shortness of breath), constipation, decreased appetite, back pain, arthralgia (joint pain), upper respiratory tract infection, pyrexia (fever), headache, abdominal pain, and vomiting.[9] Use during pregnancy may harm the baby.[1][2] Nivolumab is a human IgG4 monoclonal antibody that blocks PD-1.[2] It is a type of immunotherapy and works as a checkpoint inhibitor, blocking a signal that prevents activation of T cells from attacking the cancer.[2][3] The most common side effects when used in combination with chemotherapy include peripheral neuropathy (damage to the nerves outside of the brain and spinal cord), nausea, fatigue, diarrhea, vomiting, decreased appetite, abdominal pain, constipation and musculoskeletal pain.[8]
Nivolumab was approved for medical use in the United States in 2014.[2][6] It is on the World Health Organization's List of Essential Medicines.[10] It is made using Chinese hamster ovary cells.[11] Nivolumab is the second FDA-approved systemic therapy for mesothelioma[12] and is the first FDA-approved immunotherapy for the first-line treatment of gastric cancer.[8]
Medical uses
In the US, nivolumab is indicated to treat:
- Unresectable or Metastatic Melanoma[6]
- Adjuvant Treatment of Melanoma[6]
- Metastatic Non-Small Cell Lung Cancer[6]
- Malignant Pleural Mesothelioma[6]
- Advanced Renal Cell Carcinoma[6]
- Classical Hodgkin Lymphoma[6]
- Squamous Cell Carcinoma of the Head and Neck[6]
- Urothelial Carcinoma[6]
- Microsatellite Instability-High or Mismatch Repair Deficient Metastatic Colorectal Cancer[6]
- Hepatocellular Carcinoma[6]
- Esophageal Cancer[6]
- Gastric Cancer, Gastroesophageal Junction Cancer, and Esophageal Adenocarcinoma[6]
Nivolumab is used as a first-line treatment for inoperable or metastatic melanoma in combination with ipilimumab if the cancer does not have a mutation in BRAF,[6] and as a second-line treatment for inoperable or metastatic melanoma following treatment of ipilimumab and, if the cancer has a BRAF mutation, a BRAF inhibitor.[6][13] It is also used to treat metastatic squamous non-small cell lung cancer with progression with or after platinum-based drugs and for treatment of small cell lung cancer[6][14] It also used as a second-line treatment for renal cell carcinoma after anti-angiogenic treatment has failed.[6]
Nivolumab is used for primary or metastatic urothelial carcinoma, the most common form of bladder cancer. It can be prescribed for locally advanced or metastatic form of the condition that experience disease progression during or following platinum-containing chemotherapy or have progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.[15]
Nivolumab is indicated for the adjuvant treatment of people with melanoma with involvement of lymph nodes or in people with metastatic disease who have undergone complete resection.[6][16]
The combination of nivolumab with ipilimumab is used for the first-line treatment of adults with malignant pleural mesothelioma (MPM) that cannot be removed by surgery.[6][12]
In April 2021, the US Food and Drug Administration (FDA) approved nivolumab, in combination with certain types of chemotherapy, for the initial treatment of people with advanced or metastatic gastric cancer, gastroesophageal junction cancer and esophageal adenocarcinoma.[8]
In May 2021, the US FDA approved nivolumab for people with completely resected esophageal or gastroesophageal junction (GEJ) cancer with residual pathologic disease who have received neoadjuvant chemoradiotherapy.[9]
In August 2021, the US FDA approved nivolumab for the adjuvant treatment of people with urothelial carcinoma (UC) who are at high risk of recurrence after undergoing radical resection.[17]
In May 2022, the US FDA expanded the indication to include the first-line treatment of patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC).[18] In the same year, the FDA as approved the combination of relatlimab and nivolumab (Opdivo) for the treatment of some people with advanced melanoma.[19]
Side effects
The drug label contains warnings with regard to increased risks of severe immune-mediated inflammation of the lungs, the colon, the liver, the kidneys (with accompanying kidney dysfunction), as well as immune-mediated hypothyroidism and hyperthyroidism.[6] Hypothyroidism may affect 8.5% and hyperthyroidism 3.7%.[20] Autoimmune diabetes similar to diabetes mellitus type 1 may occur in approximately 2% of people treated with nivolumab.[20]
In trials for melanoma, the following side effects occurred in more than 10% of subjects and more frequently than with chemotherapy alone: rash and itchy skin, cough, upper respiratory tract infections, and peripheral edema. Other clinically important side effects with less than 10% frequency were ventricular arrhythmia, inflammation of parts of the eye (iridocyclitis), infusion-related reactions, dizziness, peripheral and sensory neuropathy, peeling skin, erythema multiforme, vitiligo, and psoriasis.[6]
In trials for lung cancer, the following side effects occurred in more than 10% of subjects and more frequently than with chemotherapy alone: fatigue, weakness, edema, fever, chest pain, generalized pain, shortness of breath, cough, muscle and joint pain, decreased appetite, abdominal pain, nausea and vomiting, constipation, weight loss, rash, and itchy skin.[6]
Levels of electrolytes and blood cells counts were also disrupted.[6]
Pregnancy and breastfeeding
Use during pregnancy may harm the baby.[1][6][2]
Pharmacokinetics
Based on data from 909 patients, the terminal half-life of nivolumab is 26.7 days and steady-state concentrations were reached by 12 weeks when administered at 3 mg/kg every 2 weeks.[6]:29 Age, gender, race, baseline LDH, PD-L1 expression, tumor type, tumor size, renal impairment, and mild hepatic impairment do not affect clearance of the drug.[6]:30
Mechanism of action
T cells protect the body from cancer by killing certain cancer cells. But cancer cells evolve proteins to protect themselves from T cells. Nivolumab blocks those protective proteins. Thus, the T cells can kill the cancer cells.[21][22] This is an example of immune checkpoint blockade.[21][22]
PD-1 is a protein on the surface of activated T cells. If another molecule, called programmed cell death 1 ligand 1 or programmed cell death 1 ligand 2 (PD-L1 or PD-L2), binds to PD-1, the T cell becomes inactive. This is one way that the body regulates the immune system, to avoid an overreaction.[22] Many cancer cells make PD-L1, which inhibits T cells from attacking the tumor. Nivolumab blocks PD-L1 from binding to PD-1, allowing the T cell to work.[21][22] PD-L1 is expressed on 40–50% of melanomas and has limited expression otherwise in most visceral organs with the exception of respiratory epithelium and placental tissue.[13]
Physical properties
Nivolumab is a fully human monoclonal immunoglobulin G4 antibody to PD-1.[13] The gamma 1 heavy chain is 91.8% unmodified human design while the kappa light chain is 98.9%.[23]
History
This article contains wording that promotes the subject in a subjective manner without imparting real information. (August 2023) (Learn how and when to remove this template message) |
Nivolumab was generated under intellectual property of Ono Pharmaceutical regarding PD-1 and under a research collaboration entered in 2005 between Ono and Medarex.[citation needed]
Through the research collaboration with Ono, it was invented at Medarex using its transgenic mice with a humanized immune system; the discovery and in vitro characterization of the antibody, originally called MDX-1106/ONO-4538, was published (much later) in 2014.[24] Under the agreement between the companies in 2005, Medarex held an exclusive right of nivolumab in North America, and Ono retained the right in all other countries except North America. Bristol-Myers Squibb acquired Medarex in 2009, for $2.4B, largely on the strength of its checkpoint inhibitor program.[25][26]
Promising clinical trial results made public in 2012, caused excitement among industry analysts and in the mainstream media; PD-1 was being avidly pursued as a biological target at that time, with companies including Merck with pembrolizumab (Keytruda), Roche (via its subsidiary Genentech) with atezolizumab, GlaxoSmithKline in collaboration with the Maryland biotech company Amplimmune; and Teva in collaboration with the Israeli biotech company CureTech competing.[27][28]
Ono received approval from Japanese regulatory authorities to use nivolumab to treat unresectable melanoma in July 2014, which was the first regulatory approval of a PD-1 inhibitor anywhere in the world.[29]
Merck received its first FDA approval for its PD-1 inhibitor, pembrolizumab (Keytruda), in September 2014.[30]
Nivolumab received FDA approval for the treatment of melanoma in December 2014.[13][31] In April 2015, the Committee for Medicinal Products for Human Use of the European Medicines Agency recommended approval of Nivolumab for metastatic melanoma as a monotherapy.[32]
In March 2015, the US FDA approved it for the treatment of squamous cell lung cancer.[33]
In June 2015, the European Medicines Agency (EMA) granted a marketing authorization valid throughout the European Union.[7]
In November 2015, the FDA approved nivolumab as a second-line treatment for renal cell carcinoma after having granted the application breakthrough therapy designation, fast track designation, and priority review status.[34]
In May 2016, the FDA approved nivolumab for the treatment of patients with classical Hodgkin lymphoma (cHL) who have relapsed or progressed after autologous hematopoietic stem cell transplantation (auto-HSCT) and post-transplantation brentuximab vedotin.[35]
On 20 December 2017, the FDA granted approval to nivolumab for adjuvant treatment of melanoma with involvement of lymph nodes or for metastatic disease with complete resection.[36]
On 16 April 2018, the FDA granted approval to nivolumab in combination with ipilimumab for the first-line treatment of intermediate and poor risk advanced renal cell carcinoma patients.[37]
On 15 June 2018, China's Drug Administration approved nivolumab, the country's first immuno-oncology and the first PD-1 therapy.[38]
In October 2020, the US Food and Drug Administration (FDA) approved the combination of nivolumab with ipilimumab for the first-line treatment of adults with malignant pleural mesothelioma (MPM) that cannot be removed by surgery.[12]
In April 2021, the FDA approved nivolumab, in combination with certain types of chemotherapy, for the initial treatment of people with advanced or metastatic gastric cancer, gastroesophageal junction cancer and esophageal adenocarcinoma.[8]
Research
Nivolumab, and other PD-1 inhibitors, appear to be effective in people with brain metastases[39] and for cancer in people with autoimmune diseases.[40]
Hodgkin's lymphoma
In Hodgkin's lymphoma, Reed–Sternberg cells harbor amplification of chromosome 9p24.1, which encodes PD-L1 and PD-L2 and leads to their constitutive expression. In a small clinical study published in 2015, nivolumab elicited an objective response rate of 87% in a cohort of 20 patients.[27]
The evidence is very uncertain about the effect of Nivolumab for patients with a Hodgkin's lymphoma on the overall survival, the quality of life, the survival without a progression, the response rate (=complete disappear) and grade 3 or 4 serious adverse events.[41]
Biomarkers
Amplification of chromosome 9p24 may serve as a predictive biomarker in Hodgkin's lymphoma.[27]
Each company pursuing mAbs against PD-1 as drugs developed assays to measure PD-L1 levels as a potential biomarker using their drugs as the analyte-specific reagent in the assay. Bristol Myers Squibb partnered with Dako on a nivolumab-based assay. However, as of 2015 the complexity of the immune response had hindered efforts to identify people who would be likely to respond well to PD-1 inhibitors;[27] in particular PD-L1 levels appear to be dynamic and modulated by several factors, and efforts to correlate PD-L1 levels before or during treatment with treatment response or duration of response had failed to reveal any useful correlations as of 2015.[13]
Lung cancer
In 2016, Bristol Myers Squibb announced the results of a clinical trial in which nivolumab failed to achieve its endpoint and was no better than traditional chemotherapy at treating newly diagnosed lung cancer.[42] Bristol Myers Squibb went on to attempt to win approval for a combination therapy against lung cancer which included nivolumab and Bristol Myers Squibb's older drug ipilimumab. The application was withdrawn in early 2019 following disappointing clinical trial data.[43]
Infusion times of 60 minutes and 30 minutes appear to have similar pharmacokinetics (absorption, distribution, metabolism, and elimination) of the treatment.[44]
Nivolumab is indicated for the treatment of people with metastatic squamous non small cell lung cancer (NSCLC) with progression on or after platinum-based chemotherapy.[6] CHECKMATE-227 [45] tested the combination of nivolumab and ipilimumab in patients with stage IV or recurrent NSCLC without previous treatment.[46] Patients with a PD-L1 expression level of 1% or more were randomized in a 1:1:1 ratio to receive nivolumab plus ipilimumab, nivolumab alone, or chemotherapy.[46][45] The chemotherapy used was cisplatin or carboplatin, combined with Gemcitabine for patient with squamous cell NSCLC, or pemetrexed for patients with nonsquamous disease.[45][46] The overall survival was 17.1, 15.7 and 14.9 months, respectively.[46][45] The patients who had a PD-L1 expression level of less than 1% were randomly assigned in a 1:1:1 ratio to receive nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy.[46] The OS was 17.2, 15.2 and 12.2 months, respectively[45]
In June 2023, Bristol Myers Squibb provided positive four-year follow-up results from a phase III study (CheckMate-9LA[47]) of a combination of nivolumab and ipilimumab together with chemotherapy, compared to chemotherapy alone, as a first-line treatment in patients with metastatic non-small cell lung cancer (NSCLC) showing overall survival at a minimum follow-up of 47.9 months found 21% of patients treated with the dual immunotherapy-based combination alive at four years compared to 16% of patients treated with chemotherapy alone.[47][48]
Melanoma
PD-L1 is expressed in 40-50% of melanomas.[49] Phase I and II clinical trials have shown nivolumab as a promising and durable treatment option in melanoma as a single agent and in combination with ipilimumab.[13] Phase III trials are ongoing.[50]
In October 2022, the results of a phase III trial, CheckMate -76K, showed that Opdivo reduced the risk of death by 58% as an adjuvant therapy in patients with completely resected stage two melanoma, the most serious type of skin cancer.[50][51]
Urothelial carcinoma
In February 2023, Bristol Myers Squibb reported that the three-year follow-up results from its phase III (CheckMate-274) trial of nivolumab showed significant sustained clinical benefits with nivolumab for the adjuvant treatment of patients with muscle-invasive urothelial carcinoma at a high risk of recurrence after radical resection.[52][53]
See also
- Nivolumab/relatlimab
References
- ↑ 1.0 1.1 1.2 1.3 "Nivolumab (Opdivo) Use During Pregnancy". 4 November 2019. https://www.drugs.com/pregnancy/nivolumab.html.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Nivolumab Monograph for Professionals". https://www.drugs.com/monograph/nivolumab.html.
- ↑ 3.0 3.1 "Nivolumab (Opdivo)". https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/nivolumab.
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2016". 21 June 2022. https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2016.
- ↑ "Opdivo 10 mg/mL concentrate for solution for infusion - Summary of Product Characteristics (SmPC)". 24 August 2020. https://www.medicines.org.uk/emc/product/6888.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 6.18 6.19 6.20 6.21 6.22 6.23 6.24 6.25 6.26 6.27 6.28 6.29 "Opdivo- nivolumab injection". 17 December 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f570b9c4-6846-4de2-abfa-4d0a4ae4e394.
- ↑ 7.0 7.1 7.2 7.3 "Opdivo EPAR". 30 January 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo.
- ↑ 8.0 8.1 8.2 8.3 8.4 "FDA Approves First Immunotherapy for Initial Treatment of Gastric Cancer". U.S. Food and Drug Administration (FDA) (Press release). 16 April 2021. Retrieved 16 April 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 9.0 9.1 9.2 "FDA approves nivolumab for resected esophageal or GEJ cancer". 20 May 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-resected-esophageal-or-gej-cancer.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. 2023. WHO/MHP/HPS/EML/2023.02.
- ↑ "Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers". Human Vaccines & Immunotherapeutics 12 (9): 2219–31. September 2016. doi:10.1080/21645515.2016.1175694. PMID 27135835.
- ↑ 12.0 12.1 12.2 "FDA Approves Drug Combination for Treating Mesothelioma". U.S. Food and Drug Administration (FDA) (Press release). 2 October 2020. Retrieved 2 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 "Nivolumab in melanoma: latest evidence and clinical potential". Therapeutic Advances in Medical Oncology 7 (2): 97–106. March 2015. doi:10.1177/1758834014567469. PMID 25755682.
- ↑ "Nivolumab in NSCLC: latest evidence and clinical potential". Therapeutic Advances in Medical Oncology 7 (2): 85–96. March 2015. doi:10.1177/1758834014567470. PMID 25755681.
- ↑ "Opdivo Gains FDA Approval for Common Bladder Cancer". Drug Discovery & Development. 3 February 2017. http://www.dddmag.com/article/2017/02/opdivo-gains-fda-approval-common-bladder-cancer?et_cid=5813686&et_rid=297252576&type=headline&et_cid=5813686&et_rid=297252576&linkid=content.
- ↑ "FDA grants regular approval to nivolumab for adjuvant treatment of melanoma". 20 December 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-nivolumab-adjuvant-treatment-melanoma.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "FDA approves nivolumab for adjuvant treatment of urothelial carcinoma". 20 August 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-adjuvant-treatment-urothelial-carcinoma.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "FDA approves Opdivo". 31 May 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdivo-combination-chemotherapy-and-opdivo-combination-yervoy-first-line-esophageal.
- ↑ "FDA approves nivolumab for adjuvant treatment of urothelial carcinoma". 6 April 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2022/fda-opdualag-melanoma-lag-3.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 20.0 20.1 "A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors". Hormone and Metabolic Research 51 (3): 145–156. March 2019. doi:10.1055/a-0843-3366. PMID 30861560.
- ↑ 21.0 21.1 21.2 "The blockade of immune checkpoints in cancer immunotherapy". Nature Reviews. Cancer 12 (4): 252–64. March 2012. doi:10.1038/nrc3239. PMID 22437870.
- ↑ 22.0 22.1 22.2 22.3 "De-novo and acquired resistance to immune checkpoint targeting". The Lancet. Oncology 18 (12): e731–e741. December 2017. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
- ↑ WHO Drug Information, Vol. 26, No. 2, 2012. Proposed INN List 107
- ↑ "In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates". Cancer Immunology Research 2 (9): 846–56. September 2014. doi:10.1158/2326-6066.CIR-14-0040. PMID 24872026.
- ↑ "Bristol-Myers Squibb swallows last of antibody pioneers". Nature Biotechnology 27 (9): 781–3. September 2009. doi:10.1038/nbt0909-781. PMID 19741612.
- ↑ Carroll, John (23 July 2009). "Bristol-Myers to buy Medarex for $2.1B". https://www.fiercebiotech.com/biotech/bristol-myers-to-buy-medarex-for-2-1b.
- ↑ 27.0 27.1 27.2 27.3 "The future of immune checkpoint therapy". Science 348 (6230): 56–61. April 2015. doi:10.1126/science.aaa8172. PMID 25838373. Bibcode: 2015Sci...348...56S.
- ↑ A Pollack (June 2012). "Drug helps immune system fight cancer.". New York Times. https://www.nytimes.com/2012/06/02/business/drug-helps-immune-system-fight-cancer.html.
- ↑ John Carroll for FierceBiotech 7 Jul 2014 Anti-PD-1 cancer star nivolumab wins world's first regulatory approval
- ↑ "FDA approves Keytruda for advanced melanoma" (Press release). U.S. Food and Drug Administration (FDA). 4 September 2014. Archived from the original on 25 December 2015. Retrieved 24 December 2015.
- ↑ "FDA approves Opdivo for advanced melanoma" (Press release). U.S. Food and Drug Administration (FDA). 22 December 2014. Archived from the original on 13 February 2017. Retrieved 16 December 2019.
- ↑ "New treatment for advanced melanoma". European Medicines Agency (EMA) (Press release). 24 April 2015. Retrieved 11 March 2020.
- ↑ "FDA expands approved use of Opdivo to treat lung cancer" (Press release). U.S. Food and Drug Administration (FDA). Archived from the original on 5 March 2015. Retrieved 4 March 2015.
- ↑ "FDA approves Opdivo to treat advanced form of kidney cancer". U.S. Food and Drug Administration (FDA) (Press release). 23 November 2015. Archived from the original on 25 January 2018. Retrieved 2 October 2020.
- ↑ "Nivolumab (Opdivo) for Hodgkin Lymphoma". U.S. Food and Drug Administration (FDA). 17 May 2016. https://www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-opdivo-hodgkin-lymphoma.
- ↑ "FDA grants regular approval to nivolumab for adjuvant treatment of melanoma". 21 December 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-nivolumab-adjuvant-treatment-melanoma.
- ↑ "FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma". https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-combination-intermediate-or-poor-risk-advanced-renal-cell.
- ↑ "Bristol-Myers' Opdivo ushers in a new era as the first I-O therapy approved in China | FiercePharma". 15 June 2018. https://www.fiercepharma.com/pharma-asia/opdivo-for-lung-cancer-first-i-o-therapy-approved-china.
- ↑ "Cancer immunotherapy in patients with brain metastases". Cancer Immunology, Immunotherapy 67 (5): 703–711. May 2018. doi:10.1007/s00262-018-2146-8. PMID 29520474.
- ↑ "Cancer immunotherapy in patients with preexisting autoimmune disorders". Seminars in Immunopathology 39 (3): 333–337. April 2017. doi:10.1007/s00281-016-0595-8. PMID 27730287.
- ↑ "Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer)". The Cochrane Database of Systematic Reviews 2018 (7): CD012556. July 2018. doi:10.1002/14651858.CD012556.pub2. PMID 30001476.
- ↑ "Bristol Myers: Opdivo Failed to Meet Endpoint in Key Lung-Cancer Study". Wall Street Journal. 2016-08-05. ISSN 0099-9660. https://www.wsj.com/articles/bristol-myers-opdivo-failed-to-meet-endpoint-in-key-lung-cancer-study-1470400926.
- ↑ "Bristol-Myers has Opdivo lung cancer setback as sales beat estimates". Reuters. 24 January 2019. https://finance.yahoo.com/news/bristol-myers-opdivo-lung-cancer-172155354.html.
- ↑ "Safety profile of nivolumab administered as 30-min infusion: analysis of data from CheckMate 153". Cancer Chemotherapy and Pharmacology 81 (4): 679–686. April 2018. doi:10.1007/s00280-018-3527-6. PMID 29442139.
- ↑ 45.0 45.1 45.2 45.3 45.4 "Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer". The New England Journal of Medicine 381 (21): 2020–2031. November 2019. doi:10.1056/NEJMoa1910231. PMID 31562796.
- ↑ 46.0 46.1 46.2 46.3 46.4 "First line Immunotherapy for Non-Small Cell Lung Cancer". Pharmaceuticals 13 (11): 373. November 2020. doi:10.3390/ph13110373. PMID 33171686.
- ↑ 47.0 47.1 "A Phase 3, Randomized Study of Nivolumab Plus Ipilimumab in Combination With Chemotherapy vs Chemotherapy Alone as First Line Therapy in Stage IV Non-Small Cell Lung Cancer". 2022-11-11. https://clinicaltrials.gov/ct2/show/NCT03215706.
- ↑ "Four-Year Outcomes from Phase 3 CheckMate -9LA Trial Show Durable, Long-Term Survival with Opdivo (nivolumab) Plus Yervoy (ipilimumab) with Two Cycles of Chemotherapy for Patients with Metastatic Non-Small Cell Lung Cancer". Bristol Myers Squibb (Press release). Retrieved 2023-06-05.
- ↑ "Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion". Nature Medicine 8 (8): 793–800. August 2002. doi:10.1038/nm730. PMID 12091876.
- ↑ 50.0 50.1 "CA209-76K". https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-001230-34/IT.
- ↑ "Bristol Myers Squibb Announces Adjuvant Treatment with Opdivo (nivolumab) Demonstrated Statistically Significant and Clinically Meaningful Improvement in Recurrence-Free Survival (RFS) in Patients with Stage IIB/C Melanoma in the CheckMate -76K Trial". Bristol Myers Squibb (Press release). Retrieved 2022-10-20.
- ↑ Clinical trial number NCT02632409 for "A Phase 3 Randomized, Double-blind, Multi-center Study of Adjuvant Nivolumab Versus Placebo in Subjects With High Risk Invasive Urothelial Carcinoma (CheckMate 274: CHECKpoint Pathway and nivoluMAb Clinical Trial Evaluation 274)" at ClinicalTrials.gov
- ↑ "BMS reports positive three-year results for Opdivo to treat bladder cancer". 2023-02-21. https://www.pmlive.com/pharma_news/bms_reports_positive_three-year_results_for_opdivo_to_treat_bladder_cancer_1488310.
External links
- "Nivolumab". NCI Drug Dictionary. National Cancer Institute. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/nivolumab.
- "Nivolumab". 7 January 2015. https://www.cancer.gov/about-cancer/treatment/drugs/nivolumab.
- Clinical trial number NCT01721746 for "A Study to Compare BMS-936558 to the Physician's Choice of Either Dacarbazine or Carboplatin and Paclitaxel in Advanced Melanoma Patients That Have Progressed Following Anti-CTLA-4 Therapy (CheckMate 037)" at ClinicalTrials.gov
- Clinical trial number NCT01721772 for "Study of Nivolumab (BMS-936558) Compared With Dacarbazine in Untreated, Unresectable, or Metastatic Melanoma (CheckMate 066)" at ClinicalTrials.gov
- Clinical trial number NCT01844505 for "Phase 3 Study of Nivolumab or Nivolumab Plus Ipilimumab Versus Ipilimumab Alone in Previously Untreated Advanced Melanoma (CheckMate 067)" at ClinicalTrials.gov
- Clinical trial number NCT02388906 for "Efficacy Study of Nivolumab Compared to Ipilimumab in Prevention of Recurrence of Melanoma After Complete Resection of Stage IIIb/c or Stage IV Melanoma (CheckMate 238)" at ClinicalTrials.gov
- Clinical trial number NCT02477826 for "An Investigational Immuno-therapy Trial of Nivolumab, or Nivolumab Plus Ipilimumab, or Nivolumab Plus Platinum-doublet Chemotherapy, Compared to Platinum Doublet Chemotherapy in Patients With Stage IV Non-Small Cell Lung Cancer (NSCLC) (CheckMate 227)" at ClinicalTrials.gov
- Clinical trial number NCT03215706 for "A Study of Nivolumab and Ipilimumab Combined With Chemotherapy Compared to Chemotherapy Alone in First Line NSCLC (CheckMate 9LA)" at ClinicalTrials.gov
- Clinical trial number NCT01642004 for "Study of BMS-936558 (Nivolumab) Compared to Docetaxel in Previously Treated Advanced or Metastatic Squamous Cell Non-small Cell Lung Cancer (NSCLC) (CheckMate 017)" at ClinicalTrials.gov
- Clinical trial number NCT01673867 for "Study of BMS-936558 (Nivolumab) Compared to Docetaxel in Previously Treated Metastatic Non-squamous NSCLC (CheckMate057)" at ClinicalTrials.gov
- Clinical trial number NCT02899299 for "Study of Nivolumab Combined With Ipilimumab Versus Pemetrexed and Cisplatin or Carboplatin as First Line Therapy in Unresectable Pleural Mesothelioma Patients (CheckMate743)" at ClinicalTrials.gov
- Clinical trial number NCT02231749 for "Nivolumab Combined With Ipilimumab Versus Sunitinib in Previously Untreated Advanced or Metastatic Renal Cell Carcinoma (CheckMate 214)" at ClinicalTrials.gov
- Clinical trial number NCT03141177 for "A Study of Nivolumab Combined With Cabozantinib Compared to Sunitinib in Previously Untreated Advanced or Metastatic Renal Cell Carcinoma (CheckMate 9ER)" at ClinicalTrials.gov
- Clinical trial number NCT01668784 for "Study of Nivolumab (BMS-936558) vs. Everolimus in Pre-Treated Advanced or Metastatic Clear-cell Renal Cell Carcinoma (CheckMate 025)" at ClinicalTrials.gov
- Clinical trial number NCT02181738 for "Study of Nivolumab in Patients With Classical Hodgkin's Lymphoma (Registrational) (CheckMate 205)" at ClinicalTrials.gov
- Clinical trial number NCT01592370 for "An Investigational Immuno-Therapy Study to Determine the Safety and Effectiveness of Nivolumab and Daratumumab in Patients With Multiple Myeloma" at ClinicalTrials.gov
- Clinical trial number NCT02105636 for "Trial of Nivolumab vs Therapy of Investigator's Choice in Recurrent or Metastatic Head and Neck Carcinoma (CheckMate 141)" at ClinicalTrials.gov
- Clinical trial number NCT02387996 for "A Study of Nivolumab in Participants With Metastatic or Unresectable Bladder Cancer" at ClinicalTrials.gov
- Clinical trial number NCT02060188 for "An Investigational Immuno-therapy Study of Nivolumab, and Nivolumab in Combination With Other Anti-cancer Drugs, in Colon Cancer That Has Come Back or Has Spread (CheckMate142)" at ClinicalTrials.gov
- Clinical trial number NCT01658878 for "An Immuno-therapy Study to Evaluate the Effectiveness, Safety and Tolerability of Nivolumab or Nivolumab in Combination With Other Agents in Patients With Advanced Liver Cancer (CheckMate040)" at ClinicalTrials.gov
- Clinical trial number NCT02743494 for "An Investigational Immuno-therapy Study of Nivolumab or Placebo in Participants With Resected Esophageal or Gastroesophageal Junction Cancer (CheckMate 577)" at ClinicalTrials.gov
- Clinical trial number NCT02569242 for "Study of Nivolumab in Unresectable Advanced or Recurrent Esophageal Cancer" at ClinicalTrials.gov
- Clinical trial number NCT02872116 for "Efficacy Study of Nivolumab Plus Ipilimumab or Nivolumab Plus Chemotherapy Against Chemotherapy in Stomach Cancer or Stomach/Esophagus Junction Cancer (CheckMate649)" at ClinicalTrials.gov
- Clinical trial number NCT02632409 for "An Investigational Immuno-therapy Study of Nivolumab, Compared to Placebo, in Patients With Bladder or Upper Urinary Tract Cancer, Following Surgery to Remove the Cancer (CheckMate 274)" at ClinicalTrials.gov
 |

