Chemistry:Enfortumab vedotin
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Human |
| Target | Nectin-4 |
| Clinical data | |
| Trade names | Padcev |
| Other names | AGS-22M6E, AGS-22CE, enfortumab vedotin-ejfv |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620005 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
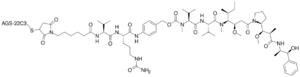
| Formula | C6642H10284N1742O2063S46 |
| Molar mass | 149024.23 g·mol−1 |
Enfortumab vedotin, sold under the brand name Padcev, is an antibody-drug conjugate[5] used for the treatment of urothelial cancer.[3][6] It is a nectin-4-directed antibody and microtubule inhibitor conjugate.[3][6] Enfortumab refers to the monoclonal antibody part, and vedotin refers to the payload drug (MMAE) and the linker.[4]
The most common side effects include fatigue, peripheral neuropathy (nerve damage resulting in tingling or numbness), decreased appetite, rash, alopecia (hair loss), nausea, altered taste, diarrhea, dry eye, pruritus (itching) and dry skin.[6]
The fully humanized antibody was created by scientists at Agensys (part of Astellas) using Xenomice from Amgen; the linker technology holding the antibody and the toxin together was provided by and licensed from Seattle Genetics.[7]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[8]
Medical uses
Enfortumab vedotin is indicated for the treatment of adults with locally advanced (when cancer has grown too large to be surgically removed) or metastatic (when cancer cells spread to other parts of the body) urothelial cancer who have previously received a programmed death receptor-1 (PD-1) or programmed death ligand 1 (PD-L1) inhibitor and a platinum-containing chemotherapy.[3][6][4]
History
Results of a Phase I clinical trial were reported in 2016.[5]
In December 2019, enfortumab vedotin was approved in the United States for the treatment of adult patients with locally advanced or metastatic urothelial cancer who had previously received a programmed cell death receptor-1 (PD-1) or programmed death ligand 1 (PD-L1) inhibitor and a platinum-containing chemotherapy.[6][9]
Enfortumab vedotin was approved based on the results of a clinical trial that enrolled 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy.[6][9] The overall response rate, reflecting the percentage of patients who had a certain amount of tumor shrinkage, was 44%, with 12% having a complete response and 32% having a partial response.[6] The median duration of response was 7.6 months.[6]
The U.S. Food and Drug Administration (FDA) granted the application for enfortumab vedotin accelerated approval, priority review designation, and breakthrough therapy designation.[6] The FDA granted the approval of Padcev to Astellas Pharma US Inc.[6]
In July 2021, the FDA approved enfortumab vedotin for adults with locally advanced or metastatic urothelial cancer who have previously received a programmed death receptor-1 (PD-1) or programmed death-ligand (PD-L1) inhibitor and platinum-containing chemotherapy, or; are ineligible for cisplatin-containing chemotherapy and have previously received one or more prior lines of therapy.[10]
Society and culture
Legal status
On 16 December 2021, and on 24 February 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Padcev, intended for the treatment of adults with urothelial cancer.[11] The applicant for this medicinal product is Astellas Pharma Europe B.V.[11] Enfortumab vedotin was approved for medical use in the European Union in April 2022.[4][12]
Names
Enfortumab vedotin is the international nonproprietary name (INN),[13] and the United States Adopted Name (USAN).[14]
References
- ↑ 1.0 1.1 "Padcev APMDS". 21 July 2022. https://www.tga.gov.au/resources/auspmd/padcev.
- ↑ "Summary Basis of Decision (SBD) for Padcev". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00573&lang=en.
- ↑ 3.0 3.1 3.2 3.3 "Padcev ejfv- enfortumab vedotin injection, powder, lyophilized, for solution". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b5631d3e-4604-4363-8f20-11dfc5a4a8ed.
- ↑ 4.0 4.1 4.2 4.3 "Padcev EPAR". 14 September 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/padcev. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 5.0 5.1 "Seattle Genetics and Agensys, an Affiliate of Astellas, Highlight Promising Enfortumab Vedotin (ASG-22ME) and ASG-15ME Phase 1 Data in Metastatic Urothelial Cancer at 2016 ESMO Congress" (Press release). Seattle Genetics and Astellas. 7 October 2016. Archived from the original on 1 April 2022. Retrieved 1 April 2022 – via Businesswire.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 6.9 "FDA approves new type of therapy to treat advanced urothelial cancer". U.S. Food and Drug Administration (FDA) (Press release). 18 December 2019. Archived from the original on 19 December 2019. Retrieved 18 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models". Cancer Research 76 (10): 3003–13. May 2016. doi:10.1158/0008-5472.can-15-1313. PMID 27013195. http://cancerres.aacrjournals.org/content/76/10/3003.long. Retrieved 19 January 2017.
- ↑ "New Drug Therapy Approvals 2019". 31 December 2019. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2019.
- ↑ 9.0 9.1 "Drug Trials Snapshots: Padcev". 13 December 2019. http://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-padcev.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "FDA grants regular approval to enfortumab vedotin-ejfv for locally advanced or metastatic urothelial cancer". 9 July 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 11.0 11.1 "Padcev: Pending EC decision". 16 December 2021. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/padcev. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Padcev Product information". https://ec.europa.eu/health/documents/community-register/html/h1615.htm.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 71". WHO Drug Information 28 (1). 2014.
- ↑ Statement On A Nonproprietary Name Adopted By The USAN Council - Enfortumab Vedotin , American Medical Association.
External links
- "Enfortumab vedotin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/enfortumab%20vedotin.
 |

