Chemistry:Rovalpituzumab tesirine
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | DLL3 |
| Clinical data | |
| ATC code |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6416H9894N1698O2028S46 (non-glycosylated) |
Rovalpituzumab tesirine (Rova-T) is an experimental antibody-drug conjugate targeting the protein DLL3 on tumor cells.[1][2] It was originally developed by Stemcentrx and was purchased by AbbVie.[3] It was tested for use in small-cell lung cancer, but development was terminated after unsuccessful phase III trial.[4][5]
Development
In 2018, an Independent Data Monitoring Committee found that in the TAHOE phase III trial, Rova-T shortened survival of lung cancer patients compared to SOC chemotherapy topotecan, prompting termination of trial enrollment. Another phase III trial (MERU) demonstrated no survival benefit over placebo.[6][7] A phase II trial using the drug as a third-line treatment for relapsed or refractory lung cancer showed objective response rate at just 16%.[8]
Chemical structure
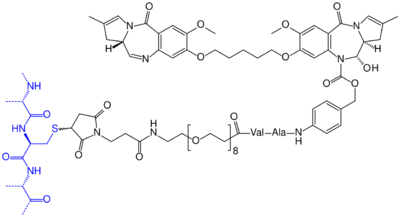
Chemical structure of "tesirine" (drawn in black). It consists of a pyrrolobenzodiazepine type dimer (top), which is the actual anti-cancer agent, a Val–Ala structure that can be cleaved by an enzyme to detach the anti-cancer agent from the antibody, a polyethylene glycol spacer, and a maleimide linker which is attached to a cysteine in the antibody's (rovalpituzumab's) peptide backbone, drawn blue. Each rovalpituzumab molecule has an average of two such attachments.[9]
See also
- Vadastuximab talirine, with a similar cytotoxin
References
- ↑ "Statement On A Nonproprietary Name Adopted By The USAN Council: USAN (de-144) Rovalpituzumab". https://searchusan.ama-assn.org/usan/documentDownload?uri=%2Funstructured%2Fbinary%2Fusan%2Frovalpituzumab.pdf.
- ↑ "Rovalpituzumab Tesirine: A Novel DLL3-Targeting Antibody-Drug Conjugate". Drugs in R&D 18 (4): 255–258. December 2018. doi:10.1007/s40268-018-0247-7. PMID 30232719.
- ↑ "Rova-T (Rovalpituzumab tesirine)". BioCentury. http://bciq.biocentury.com/products/rova-t.
- ↑ "AbbVie Discontinues Rovalpituzumab Tesirine (Rova-T) Research and Development Program | AbbVie News Center". https://news.abbvie.com/news/press-releases/abbvie-discontinues-rovalpituzumab-tesirine-rova-t-research-and-development-program.htm.
- ↑ Alternative Names: Rova-T; SC16LD6.5. "Rovalpituzumab tesirine - AdisInsight". Adisinsight.springer.com. http://adisinsight.springer.com/drugs/800038447.
- ↑ "It's official—AbbVie dumps Rova-T after another lung cancer fail" (in en). https://www.fiercebiotech.com/biotech/it-s-official-abbvie-dumps-rova-t-after-another-lung-cancer-flop.
- ↑ Clinical trial number NCT03033511 for "A Study of Rovalpituzumab Tesirine as Maintenance Therapy Following First-Line Platinum-Based Chemotherapy in Participants With Extensive Stage Small Cell Lung Cancer (MERU) " at ClinicalTrials.gov
- ↑ "AbbVie ditches plans for accelerated Rova-T review after weak phase 2 data" (in en). https://www.fiercebiotech.com/biotech/abbvie-ditches-plans-for-accelerated-rova-t-review-after-weak-phase-2-data.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended INN: List 75". WHO Drug Information (World Health Organization) 30 (1): 151. 2016. https://www.who.int/entity/medicines/publications/druginformation/innlists/RL75.pdf.
 |
