Chemistry:Dinitrogen difluoride
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
cis- or trans-dinitrogen difluoride
| |||
| Other names
cis- or trans-difluorodiazene
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| FN=NF | |||
| Molar mass | 66.011 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 2.698 g/L | ||
| Melting point | cis: less than −195 °C (−319.0 °F; 78.1 K) trans: −172 °C (−278 °F) | ||
| Boiling point | cis: −105.75 °C (−158.35 °F; 167.40 K) trans: −111.45 °C (−168.61 °F) | ||
| cis: 0.16 D trans: 0 D | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
cis: 69.5 kJ/mol trans: 82.0 kJ/mol | ||
| Related compounds | |||
Other anions
|
Azide | ||
Other cations
|
| ||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
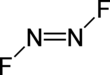
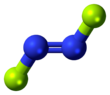
Dinitrogen difluoride is a chemical compound with the formula N
2F
2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the fluorine azide (FN
3). It has the structure F–N=N–F and exists in both cis and trans isomers, as typical for diimides.
Isomers
The cis isomer has C2v symmetry and the trans isomer has C2h symmetry. These isomers can interconvert, but the process is slow enough at low temperature that the two can separated by low-temperature fractionation.[clarification needed] The trans isomer is less thermodynamically stable[citation needed] but can be stored in glass vessels. The cis isomer attacks glass over a time scale of about 2 weeks to form silicon tetrafluoride and nitrous oxide:[2][page needed]
- 2 N
2F
2 + SiO
2 → SiF
4 + 2 N
2O
Preparation
Most preparations of dinitrogen difluoride give mixtures of the two isomers, but they can be prepared independently.
An aqueous method involves N,N-difluorourea with concentrated potassium hydroxide. This gives a 40% yield with three times more of the trans isomer.[3]
Difluoramine forms a solid unstable compound with potassium fluoride (or rubidium fluoride or caesium fluoride) which decomposes to dinitrogen difluoride.[3]
It can also be prepared by photolysis of tetrafluorohydrazine and bromine:[4]
- N
2F
4 N
2F
2 + byproducts
Reactions
The cis form of difluorodiazene will react with strong fluoride ion acceptors such as antimony pentafluoride to form the linear[5] [N≡N–F]+
cation (fluorodiazonium cation[5]) which forms a salt with the formula [N≡N–F]+
[SbF
6]−
(fluorodiazonium hexafluoroantimonate(V)).
- F–N=N–F + SbF
5 → [N≡N–F]+
[SbF
6]−
Analogous reaction of cis-difluorodiazene with arsenic pentafluoride gives white solid salt with the formula [N≡N–F]+
[AsF
6]−
[5] (fluorodiazonium hexafluoroarsenate(V)).
- F–N=N–F + AsF
5 → [N≡N–F]+
[AsF
6]−
In the solid phase, the observed N≡N and N–F bond distances in the [N≡N–F]+
cation are 1.089(9) and 1.257(8) Å respectively, among the shortest experimentally observed N-N and N-F bonds.
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. pp. 4–73, 5–15, 9–46. ISBN 0-8493-0594-2.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 3.0 3.1 Sykes, A. G. (1989-07-17). Advances in Inorganic Chemistry. Academic Press. p. 171. ISBN 9780080578828. https://books.google.com/books?id=qzN5pnPwuaoC&pg=PA171. Retrieved 21 June 2014.
- ↑ Leon M. Zaborowski (1973) (in German), Chlorodifluoroamine and Difluorodiazene - B. Difluorodiazene (Dinitrogen difluoride), Inorganic Syntheses, 14, McGraw-Hill Book Company, Inc., pp. 34–39
- ↑ 5.0 5.1 5.2 Cacace, Fulvio; Grandinetti, Felice; Pepi, Federico (1995). "Gaseous Fluorodiazonium Ions. Experimental and Theoretical Study on Formation and Structure of FN2+". Inorganic Chemistry 34 (6): 1325–1332. doi:10.1021/ic00110a007. https://pubs.acs.org/doi/10.1021/ic00110a007.
 |