Chemistry:Tetrafluorohydrazine
| |
| |
| Names | |
|---|---|
| IUPAC name
1,1,2,2-tetrafluorohydrazine
| |
| Other names
Tetrafluorohydrazine, perfluorohydrazine, UN 1955
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
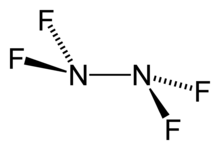
| N 2F 4 | |
| Molar mass | 104.008 g·mol−1 |
| Appearance | Colourless gas |
| Melting point | −164.5 °C (−264.1 °F; 108.6 K)[1] |
| Boiling point | −73 °C (−99 °F; 200 K)[1] |
| Hazards | |
| Main hazards | Explosion |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
10 mL/kg (rat, intraperitoneal)[2] |
LC50 (median concentration)
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrafluorohydrazine or perfluorohydrazine, N
2F
4, is a colourless, nonflammable,[2] reactive inorganic gas. It is a fluorinated analog of hydrazine.
Tetrafluorohydrazine is manufactured from nitrogen trifluoride using an iron catalyst or iron(II) fluoride.
Properties
Tetrafluorohydrazine is in equilibrium with its radical monomer nitrogen difluoride.[3]
- N
2F
4 ⇌ 2 •NF
2
At room temperature N
2F
4 is mostly associated with only 0.7% in the form of NF
2 at 5mm Hg pressure. When the temperature rises to 225 °C, it mostly dissociates with 99% in the form of NF
2.[4]
The energy needed to break the N-N bond in N
2F
4 is 20.8 kcal/mol, with an entropy change of 38.6 eu.[4] For comparison, the dissociation energy of the N-N bond is 14.6 kcal/mol in N
2O
4, 10.2 kcal/mol in N
2O
2, and 60 kcal/mol in N
2H
4. The enthalpy of formation of N
2F
4 (ΔHf) is 34.421 kJ/mol.[5]
Uses
Tetrafluorohydrazine is used in some chemical syntheses, as a precursor or a catalyst. It was considered for use as a high-energy liquid oxidizer in some never-flown rocket fuel formulas in 1959.[6] Tetrafluorohydrazine is used in organic synthesis and as an oxidizing agent for rocket fuels.[2]
Safety
Tetrafluorohydrazine is a highly hazardous chemical that explodes in the presence of organic materials.[2]
It is a toxic chemical which irritates skin, eyes and lungs. It is a neurotoxin and may cause methemoglobinemia. May be fatal if inhaled or absorbed through skin. Vapors may be irritating and corrosive. It is a strong oxidizing agent. Contact with this chemical may cause burns and severe injury. Fire produces irritating, corrosive and toxic gases. Vapors from liquefied gas are initially heavier than air and spread across the ground.[2]
Tetrafluorohydrazine explodes or ignites on contact with reducing agents at room temperature, including hydrogen, hydrocarbons, alcohols, thiols, amines, ammonia, hydrazines, dicyanogen, nitroalkanes, alkylberylliums, silanes, boranes or powdered metals. Prolonged exposure of the container of tetrafluorohydrazine to high heat may cause it to rupture violently and rocket. Tetrafluorohydrazine itself can explode at high temperatures or with shock or blast when under pressure. When heated to decomposition in air, it emits highly toxic fumes of fluorine and oxides of nitrogen.[2]
There is a fatal case in which during opening of valves to check the pressure, the cylinder exploded, killing one man and injuring another.[2]
References
- ↑ 1.0 1.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Tetrafluorohydrazine" (in en). National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/Tetrafluorohydrazine.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Jäger, Susanne; von Jouanne, Jörn; Keller-Rudek, Hannelore; Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Rupecht, Sigrid; Vanecek, Hans et al. (1986). Koschel, Dieter; Kuhn, Peter; Merlet, Peter et al.. eds. F Fluorine: Compounds with Oxygen and Nitrogen. Gmelin Handbook of Inorganic Chemistry. 4. Berlin: Springer. p. 162. doi:10.1007/978-3-662-06339-2. ISBN 978-3-662-06341-5. https://books.google.com/books?id=rpfsCAAAQBAJ&pg=PA162. Retrieved 29 August 2015.
- ↑ 4.0 4.1 Bohn, Robert K.; Bauer, Simon Harvey (February 1967). "An electron diffraction study of the structures of NF2 and N2F4". Inorganic Chemistry 6 (2): 304–309. doi:10.1021/ic50048a024. molecule dimensions and angles
- ↑ "Nitrogen difluoride NF2(g)". http://www.chem.msu.su/rus/handbook/ivtan/100.html.
- ↑ Tetrafluorohydrazine at DTIC.mil archived March 12, 2007
 |

