Chemistry:Tetrazene
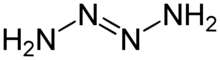
| |
| Names | |
|---|---|
| IUPAC name
(2E)-2-Tetraazene
| |
| Other names
(2E)-2-Tetraazen; Tetraaz-1-ene
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| H4N4 | |
| Molar mass | 60.060 g·mol−1 |
| Related compounds | |
Related binary azanes
|
Ammonia Hydrazine Triazane |
Related compounds
|
Diazene Triazene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrazene is a chemical compound with the molecular formula H2NN=NNH2. It is a colorless explosive material. An analogue is the organosilicon derivative (tms)2NN=NN(tms)2 where tms is trimethylsilyl.[1] Isomeric with tetrazine is ammonium azide.
Tetrazene explosive, commonly known simply as tetrazene, is used for sensitization of priming compositions.
Properties
Tetrazene has eleven isomers.[2] The most stable of these is the straight-chain 2-tetrazene (H2N-N=N-NH2), having a standard heat of formation at 301.3 kJ/mol. The eleven isomers can be arranged into three groups: straight-chain tetrazenes, four-membered cyclotetrazane, and three-membered cyclotriazanes. Each straight-chain tetrazene isomer possesses one N=N double bond and two N-N single bonds.[2] Tautomerizations do occur between the isomers. The ionic compound ammonium azide is also a constitutional isomer of tetrazene.
Organometallic derivatives
A variety of coordination complexes are known for R2N42- (R = methyl, benzyl).[3]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 2.0 2.1 Li, L.-C.; Shang, J.; Liu, J.-L.; Wang, X.; Wong, N.-B. (2007). "A G3B3 study of N4H4 isomers". Journal of Molecular Structure 807 (1–3): 207–10. doi:10.1016/j.theochem.2006.12.009.
- ↑ Bowman, Amanda C.; Tondreau, Aaron M.; Lobkovsky, Emil; Margulieux, Grant W.; Chirik, Paul J. (2018). "Synthesis and Electronic Structure Diversity of Pyridine(diimine)iron Tetrazene Complexes". Inorganic Chemistry 57 (16): 9634–9643. doi:10.1021/acs.inorgchem.8b00140. PMID 29620870.
 |







