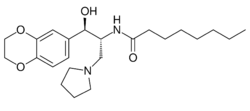
Chemistry:Eliglustat
 | |
| Clinical data | |
|---|---|
| Trade names | Cerdelga |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618038 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H36N2O4 |
| Molar mass | 404.551 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Eliglustat, sold under the brand name Cerdelga, is a medication used for the treatment of Gaucher's disease. It was discovered at the University of Michigan, developed by Genzyme Corp, and was approved by the FDA in August 2014.[4] Commonly used as the tartrate salt, the compound is believed to work by inhibition of glucosylceramide synthase.[5][6] According to an article in Journal of the American Medical Association the oral substrate reduction therapy resulted in "significant improvements in spleen volume, hemoglobin level, liver volume, and platelet count" in untreated adults with Gaucher disease Type 1.[7]
History
Norman Radin began exploring the possibility of inhibiting the synthesis of lipid substrates involved in Gaucher's disease as early as 1982, and, in collaboration with the laboratory of Jim Shayman, found several candidate inhibitors in the mid-1990s.[8] Genzyme initially rejected the candidates developed by Radin and Shayman, but after a news broke of a competitor developing a new treatment for Gaucher's disease, licensed the Radin/Shayman patents in 2000.[8] Eliglustat did not receive FDA approval for another 14 years, a delay that Shayman speculated was due to some company leaders not being fully committed to developing a drug that would compete with imiglucerase (brand name Cerezyme), Genzyme's flagship treatment for Gaucher's disease.[8]
Society and culture
Economics
In 2014, the annual cost of eliglustat taken orally twice a day was $310,250. Cerezyme cost about $300,000 for the intravenous medication if taken twice a month.[9] Manufacturing costs for eliglustat are slightly lower than for imiglucerase. Genzyme maintains higher prices for orphan drugs—most often paid for by insurers—in order to remain financially sustainable.[9]
References
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2015". 21 June 2022. https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2015.
- ↑ "Cerdelga 84 mg Hard Capsules - Summary of Product Characteristics (SmPC)". 8 January 2020. https://www.medicines.org.uk/emc/product/2615.
- ↑ "Cerdelga- eliglustat capsule". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=819f828a-b888-4e46-83fc-94d774a28a83.
- ↑ "FDA approves new drug to treat a form of Gaucher disease" (Press release). U.S. Food and Drug Administration. 19 August 2015. Archived from the original on 16 February 2017. Retrieved 18 July 2015.
- ↑ "Improved inhibitors of glucosylceramide synthase". The Journal of Biological Chemistry 274 (21): 14662–9. May 1999. doi:10.1074/jbc.274.21.14662. PMID 10329660.
- ↑ "ELIGLUSTAT TARTRATE: Glucosylceramide Synthase Inhibitor Treatment of Type 1 Gaucher Disease". Drugs of the Future 35 (8): 613–620. August 2010. doi:10.1358/dof.2010.035.08.1505566. PMID 22563139.
- ↑ "Effect of oral eliglustat on splenomegaly in patients with Gaucher disease type 1: the ENGAGE randomized clinical trial". JAMA 313 (7): 695–706. February 2015. doi:10.1001/jama.2015.459. PMID 25688781.
- ↑ 8.0 8.1 8.2 Garber, Ken (December 2017), Just Reward, Ann Arbor Observer, https://annarborobserver.com/articles/just_reward_full_article.html, retrieved 17 January 2021
- ↑ 9.0 9.1 Weisman, Robert (2 September 2014), New Genzyme pill will cost patients $310,250 a year, The Boston Globe, https://www.bostonglobe.com/business/2014/09/02/new-genzyme-pill-treat-rare-gaucher-disease-will-cost-patients-year/5thkIb587nKi7zRAb9GgxM/story.html, retrieved 18 July 2015
External links
- "Eliglustat". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/eliglustat.
 |

