Chemistry:Miglustat
 | |
| Clinical data | |
|---|---|
| Trade names | Zavesca, Brazaves, Opfolda |
| Other names | OGT 918, 1,5-(butylimino)-1,5-dideoxy-D-glucitol, N-butyl-deoxynojirimycin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604015 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 97% |
| Protein binding | Nil |
| Metabolism | Nil |
| Elimination half-life | 6–7 hours |
| Excretion | Kidney, unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
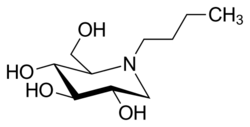
| Formula | C10H21NO4 |
| Molar mass | 219.281 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Miglustat, sold under the brand name Zavesca among others, is a medication used to treat type I Gaucher disease[7] and Pompe disease.[10]
It was approved for medical use in the European Union in November 2002,[7][11] and for medical use in the United States in July 2003.[12][13]
Medical uses
Miglustat is indicated to treat adults with mild to moderate type I Gaucher disease for whom enzyme replacement therapy is unsuitable.[14]
In the European Union, miglustat (Opfolda), in combination with cipaglucosidase alfa, is a long-term enzyme replacement therapy in adults with late-onset Pompe disease (acid α‑glucosidase [GAA] deficiency).[10]
Contraindications
Miglustat is contraindicated for people with neurological conditions, kidney problems, women who are pregnant, and men and women planning to conceive a child.[15]
Adverse effects
Serious side effects include pain, burning, numbness or tingling in the hands, arms, legs, or feet; shaking hands that cannot be controlled; changes in vision; and easy bruising or bleeding. Common side effects include gastrointestinal effects (including diarrhea, stomach pain or bloating, gas, loss of appetite, weight loss, upset stomach, vomiting, constipation), dry mouth, muscular effects (including weakness, muscle cramps, especially in the legs, feeling of heaviness in the arms or legs, unsteadiness when walking), back pain, dizziness, nervousness, headache, memory problems, and difficult or irregular menstruation (period).[15]
Mechanism of action
Type I Gaucher's disease is an autosomal recessive disorder; parents are generally healthy carriers with one functional and one mutated (nonfunctioning) copy of the Gaucher disease gene, GBA. People with type I Gaucher have a defect in the enzyme called glucocerebrosidase (also known as acid β-glucosidase). Glucocerebrosidase is an enzyme, and its function is to convert glucocerebroside (also known as glucosylceramide) into ceramide and glucose. When this enzyme doesn't work, glucocerebroside accumulates, which in turn causes liver and spleen enlargement, changes in the bone marrow and blood, and bone disease. Miglustat functions as a competitive and reversible inhibitor of the enzyme glucosylceramide synthase, the initial enzyme in a series of reactions which results in the synthesis of most glycosphingolipids.[16][17]
Earlier treatments on the market (imiglucerase (approved in 1995),[18] velaglucerase (approved in 2010),[19] taliglucerase alfa (Elelyso) (approved in 2012)[20]) are enzyme replacement therapy—they are functioning versions of the enzyme that doesn't work. Miglustat, on the other hand, prevents the formation of the substance that builds up when the enzyme doesn't work; this is called substrate reduction therapy.[21]
Chemistry
Miglustat is an iminosugar, a synthetic analogue of D-glucose[22] and a white to off-white crystalline solid that has a bitter taste.[23]
Society and culture
Legal status
Miglustat has been approved in the EU, Canada, and Japan for treating progressive neurological complications in people with Niemann–Pick disease, type C (NPC).[24][25][26][27][28]
On 26 April 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Opfolda, intended for the treatment of glycogen storage disease type II (Pompe disease) in combination with cipaglucosidase alfa.[10] The applicant for this medicinal product is Amicus Therapeutics Europe Limited.[10] Opfolda is a hybrid medicine of Zavesca which has been authorized in the EU since 2002.[10] Opfolda contains the same active substance as Zavesca but in a lower strength.[10] It is also authorized for a different indication and can only be used in combination with cipaglucosidase alfa.[10] Miglustat (Opfolda) was approved for medical use in the European Union in June 2023.[8][29]
Research
In July 2004, Actelion started a clinical trial of miglustat to treat Tay–Sachs disease, particularly late-onset Tay–Sachs with an estimated enrollment of 10 subjects; the trial ended August 2007.[30]
In November 2007, Actelion initiated a clinical trial with miglustat in people with cystic fibrosis (CF) who have the ΔF508 in both copies of the cystic fibrosis transmembrane conductance regulator (CFTR) gene; the study ended in March 2008.[31] The cystic fibrosis trial showed no effect.[32]
N-butyldeoxynojirimycin interferes with the secretion of hepatitis B virus[33] and reduces the infectivity of HIV virions, in the latter case by preventing proper folding of the gp160 precursor glycoprotein to cause a conformational defect in mature gp120, which interferes with the process of fusion with host membranes.[34][35]
References
- ↑ "Miglustat (Zavesca) Use During Pregnancy". 4 February 2020. https://www.drugs.com/pregnancy/miglustat.html.
- ↑ "Summary for ARTG Entry:122957 Zavesca miglustat 100 mg capsules blister pack". http://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=1C58E66A2138FA60CA2585880030F0B2&agid=(PrintDetailsPublic)&actionid=1.[yes|permanent dead link|dead link}}]
- ↑ "Zavesca (miglustat) 100 mg hard capsules - Summary of Product Characteristics (SmPC)". https://www.medicines.org.uk/emc/product/39/smpc.
- ↑ "Zavesca- miglustat capsule". 20 April 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=817892d1-ee12-4632-85fc-57ccdf16d7b8.
- ↑ "Yargesa- miglustat capsule". 12 July 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2b371dfe-e2b0-47fe-b677-a7bde7149f87.
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215211s000lbl.pdf
- ↑ 7.0 7.1 7.2 "Zavesca EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/zavesca.
- ↑ 8.0 8.1 "Opfolda EPAR". 7 July 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/opfolda.
- ↑ "Yargesa EPAR". 11 April 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/yargesa.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 "Opfolda: Pending EC decision". 26 April 2023. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/opfolda. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ European Medicines Agency. Human Medicines Database. Zavesca (miglustat) Page Accessed 1 September 2014.
- ↑ "Drug Approval Package: Zavesca (Miglustat) NDA #021348". 4 April 2002. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-348_Zavesca.cfm.
- ↑ Actelion Press Release August 2003. Zavesca approved -- first oral treatment option for type 1 Gaucher disease
- ↑ "The role of the iminosugar N-butyldeoxynojirimycin (miglustat) in the management of type I (non-neuronopathic) Gaucher disease: a position statement". Journal of Inherited Metabolic Disease 26 (6): 513–526. 2003. doi:10.1023/a:1025902113005. PMID 14605497.
- ↑ 15.0 15.1 American Society of Health-System Pharmacists, Inc. for the Public Library of Medicine. Miglustat on MedlinePlus Accessed 1 September 2014
- ↑ "Gaucher disease and other storage disorders". Hematology. American Society of Hematology. Education Program 2012: 13–18. 2012. doi:10.1182/asheducation.v2012.1.13.3797921. PMID 23233555.
- ↑ "FDA Clinical Investigator Site Inspections: The Sponsor's Role". Drug Information Journal 33 (3): 965–968. July 1999. doi:10.1177/009286159903300338. ISSN 0092-8615.
- ↑ "Imiglucerase in the treatment of Gaucher disease: a history and perspective". Drug Design, Development and Therapy 6: 81–106. 2012. doi:10.2147/DDDT.S14395. PMID 22563238.
- ↑ "Shire Announces FDA Approval Of VPRIV(TM) (velaglucerase Alfa For Injection) For The Treatment Of Type I Gaucher Disease". Medicalnewstoday.com. http://www.medicalnewstoday.com/articles/180630.php.
- ↑ "U.S. FDA approves Pfizer/Protalix drug for Gaucher". Chicago Tribune. Reuters. 1 May 2012. http://www.chicagotribune.com/health/sns-rt-us-fda-gaucherbre8401jz-20120501,0,5155428.story.[yes|permanent dead link|dead link}}]
- ↑ Actelion. FDA Advisory Briefing Book for Miglustat (Ogt 918, Zavesca) in Niemann-Pick Type C Disease NDA 021-348/S-007 Prepared for the Endocrinologic and Metabolic Drugs Advisory Committee meeting, 1 December 2009
- ↑ "Therapeutic strategies for Gaucher disease: miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerase alfa and imiglucerase". Molecular Pharmaceutics 8 (6): 2390–2397. December 2011. doi:10.1021/mp200313e. PMID 21988669.
- ↑ European Medicines Agency 1 April 2003 Scientific discussion related to approval of Zavesca .
- ↑ UK Medicines Information. New Drugs Online Report for miglustat
- ↑ Staff, The Pharma Letter. 4 April 2012. Actelion drops setipiprant, gets miglustat approval in Japan
- ↑ Kevin Grogan for PharmaTimes. 10 March 2010. FDA rejects Actelion's Zavesca for rare NP-C disease
- ↑ Actelion Press Release. 23 March 2010 Zavesca (Miglustat) First Treatment Available in Canada for Rare Progressive Niemann-Pick Type C Disease
- ↑ "Association of Miglustat With Swallowing Outcomes in Niemann-Pick Disease, Type C1". JAMA Neurology 77 (12): 1564–1568. December 2020. doi:10.1001/jamaneurol.2020.3241. PMID 32897301.
- ↑ "Opfolda Product information". 27 June 2023. https://ec.europa.eu/health/documents/community-register/html/h1737.htm.
- ↑ Clinical trial number NCT00672022 for "Pharmacokinetics, Safety and Tolerability of Zavesca (Miglustat) in Patients With Infantile Onset Gangliosidosis: Single and Steady State Oral Doses" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00537602 for "OGT 918 in the Treatment of Cystic Fibrosis" at ClinicalTrials.gov
- ↑ "A randomized placebo-controlled trial of miglustat in cystic fibrosis based on nasal potential difference". Journal of Cystic Fibrosis 11 (3): 231–236. May 2012. doi:10.1016/j.jcf.2011.12.004. PMID 22281182.
- ↑ "Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin". Proceedings of the National Academy of Sciences of the United States of America 91 (6): 2235–2239. March 1994. doi:10.1073/pnas.91.6.2235. PMID 8134380. Bibcode: 1994PNAS...91.2235B.
- ↑ "N-butyldeoxynojirimycin-mediated inhibition of human immunodeficiency virus entry correlates with changes in antibody recognition of the V1/V2 region of gp120". Journal of Virology 70 (10): 7143–7152. October 1996. doi:10.1128/JVI.70.10.7143-7152.1996. PMID 8794361.
- ↑ "N-Butyldeoxynojirimycin is a broadly effective anti-HIV therapy significantly enhanced by targeted liposome delivery". AIDS 22 (15): 1961–1969. October 2008. doi:10.1097/QAD.0b013e32830efd96. PMID 18753929.
 |

