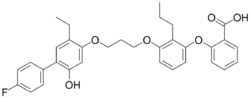
Chemistry:Etalocib
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
25-Ethyl-14-fluoro-22-hydroxy-82-propyl-3,7,9-trioxa-1,10(1),2(1,4),8(1,3)-tetrabenzenadecaphane-102-carboxylic acid | |
| Other names
LY293111
VML 295 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C33H33FO6 | |
| Molar mass | 544.619 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Etalocib is a drug candidate that was under development for the treatment of various types of cancer.[1] It acts as a leukotriene B4 receptor antagonist and a PPARγ agonist.[2]
Clinical trials were conducted measuring efficacy for treatment of non-small cell lung cancer and pancreatic cancer and the inflammatory conditions asthma, psoriasis, and ulcerative colitis, but were suspended due to lack of efficacy.[3]
References
- ↑ Jänne, P. A.; Paz-Ares, L; Oh, Y; Eschbach, C; Hirsh, V; Enas, N; Brail, L; von Pawel, J (2014). "Randomized, double-blind, phase II trial comparing gemcitabine-cisplatin plus the LTB4 antagonist LY293111 versus gemcitabine-cisplatin plus placebo in first-line non-small-cell lung cancer". Journal of Thoracic Oncology 9 (1): 126–31. doi:10.1097/JTO.0000000000000037. PMID 24346102.
- ↑ Adrian, T. E.; Hennig, R; Friess, H; Ding, X (2008). "The Role of PPARgamma Receptors and Leukotriene B(4) Receptors in Mediating the Effects of LY293111 in Pancreatic Cancer". PPAR Research 2008: 827096. doi:10.1155/2008/827096. PMID 19190780.
- ↑ "Etalocib". http://adisinsight.springer.com/drugs/800003262.
 |

