Chemistry:Roflumilast
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Daxas, Daliresp, Zoryve, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611034 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 79%[2][1][5][6] |
| Protein binding | 99%[2][1][5][6] |
| Metabolism | Hepatic via CYP1A2 & CYP3A4[2][1][5][6] |
| Elimination half-life | 17 hours (30 hours [active metabolite])[2][1][5][6] |
| Excretion | Urine (70%)[2][1][5][6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
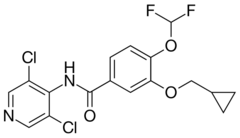
| Formula | C17H14Cl2F2N2O3 |
| Molar mass | 403.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Roflumilast, sold under the brand name Daxas among others, is a medication that acts as a selective, long-acting inhibitor of the enzyme phosphodiesterase-4 (PDE-4). It has anti-inflammatory effects and is used as an orally administered drug for the treatment of inflammatory conditions of the lungs such as chronic obstructive pulmonary disease (COPD).[7][8][9][10]
In June 2010, it was approved in the European Union for severe COPD associated with chronic bronchitis.[4][11] In February 2011, it gained FDA approval in the United States for reducing COPD exacerbations.[12][13] It is available as a generic medication.[14]
Medical uses
Roflumilast is indicated for the treatment of severe chronic obstructive pulmonary disease (COPD)[2] and for the treatment of plaque psoriasis.[3][15]
It is used in the prevention of exacerbations (lung attacks) in severe chronic obstructive pulmonary disease (COPD).[1][2][4][5][6]
Adverse effects
Common (1–10% incidence) adverse effects include diarrhea, weight loss, nausea, headache, insomnia, decreased appetite, abdominal pain, rhinitis, sinusitis, urinary tract infection, and depression.[2][1][5][6][16]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Daxas 250 micrograms tablets - Summary of Product Characteristics (SmPC)". 11 June 2020. https://www.medicines.org.uk/emc/product/9162/smpc.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 "Daliresp- roflumilast tablet". 12 March 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c9a1d0a8-581f-4f91-a2b8-f419192d0ebf.
- ↑ 3.0 3.1 "Zoryve- roflumilast cream". 16 August 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ec1bb0d1-f38a-4080-831a-68791d1d1fdb.
- ↑ 4.0 4.1 4.2 "Daxas EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/daxas.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 "Daliresp : EPAR - Product Information". European Medicines Agency. Takeda GmbH. 26 September 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002398/WC500103078.pdf.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 "roflumilast (Rx) - Daliresp". Medscape Reference. WebMD. http://reference.medscape.com/drug/daliresp-roflumilast-999626.
- ↑ "PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast". International Journal of Chronic Obstructive Pulmonary Disease 2 (2): 121–9. 2007. PMID 18044684.
- ↑ "Suppression of cytokine expression by roflumilast and dexamethasone in a model of chronic asthma". Clinical and Experimental Allergy 38 (5): 847–56. May 2008. doi:10.1111/j.1365-2222.2008.02950.x. PMID 18307529.
- ↑ "Roflumilast attenuates pulmonary inflammation upon segmental endotoxin challenge in healthy subjects: a randomized placebo-controlled trial". Pulmonary Pharmacology & Therapeutics 21 (4): 616–23. August 2008. doi:10.1016/j.pupt.2008.02.002. PMID 18374614. https://hal.archives-ouvertes.fr/hal-00499153/file/PEER_stage2_10.1016%252Fj.pupt.2008.02.002.pdf. Retrieved 24 June 2019.
- ↑ "Roflumilast: an oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma". Expert Opinion on Investigational Drugs 17 (5): 811–8. May 2008. doi:10.1517/13543784.17.5.811. PMID 18447606.
- ↑ ""Nycomed's Anti-Inflammatory Gains Approval in EU for COPD"". http://www.genengnews.com/gen-news-highlights/nycomed-s-anti-inflammatory-gains-approval-in-eu-for-copd/81243622/.
- ↑ "Drug Approval Package: Daliresp Tablets (roflumilast) NDA #022522". 24 December 1999. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022522Orig1s000TOC.cfm.
- ↑ "FDA approves new drug to treat chronic obstructive pulmonary disease" (Press release). U.S. Food and Drug Administration (FDA). 1 March 2011. Archived from the original on 18 January 2017. Retrieved 16 December 2019.
- ↑ "2022 First Generic Drug Approvals". 3 March 2023. https://www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/2022-first-generic-drug-approvals.
- ↑ "FDA Approves Arcutis' Zoryve (Roflumilast) Cream 0.3% For the Treatment of Plaque Psoriasis in Individuals Age 12 and Older" (Press release). Arcutis Biotherapeutics. 29 July 2022. Archived from the original on 1 August 2022. Retrieved 1 August 2022 – via GlobeNewswire.
- ↑ "PDE4 inhibitors: current status". British Journal of Pharmacology 155 (3): 308–15. October 2008. doi:10.1038/bjp.2008.307. PMID 18660825.
 |

