Chemistry:Abediterol
From HandWiki
Short description: Chemical compound
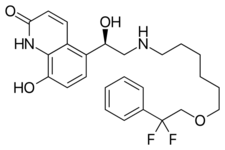 | |
| Clinical data | |
|---|---|
| Routes of administration | Inhalation |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | 24.3 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H30F2N2O4 |
| Molar mass | 460.522 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Abediterol (INN;[2] development codes AZD-0548 and LAS 100977) is a once-daily experimental drug candidate for the treatment of asthma and chronic obstructive pulmonary disease (COPD). It is currently under development by the Spanish pharmaceutical company Almirall and is in Phase II clinical trials.[3][4]
It acts as a dual β2 adrenergic agonist[5][6] and muscarinic antagonist and is classified as an ultra-long-acting β2 agonist (ultra-LABA).[7]
Its coformulation with mometasone furoate is also in Phase II clinical trials.[8][when?][needs update]
References
- ↑ "First-in-human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of abediterol (LAS100977), a novel long-acting Β2 -agonist". Journal of Clinical Pharmacology 54 (12): 1347–53. December 2014. doi:10.1002/jcph.355. PMID 24989946.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed International Nonproprietary Names: List 104". WHO Drug Information 24 (1): 352. 2010. https://www.who.int/medicines/publications/druginformation/issues/PL-104.pdf. Retrieved 25 March 2016.
- ↑ Product development pipeline, Almirall
- ↑ "AdisInsight: Abediterol". Springer International Publishing AG. http://adisinsight.springer.com/drugs/800026746.
- ↑ "Espacenet - Bibliographic data". https://worldwide.espacenet.com/publicationDetails/biblio?CC=WO&NR=2006122788&KC=&FT=E&locale=en_EP.
- ↑ "Espacenet - Bibliographic data". https://worldwide.espacenet.com/publicationDetails/biblio?CC=WO&NR=2010094484&KC=&FT=E&locale=en_EP.
- ↑ "Abediterol (LAS100977), a novel long-acting β2-agonist: efficacy, safety and tolerability in persistent asthma". Respiratory Medicine 108 (10): 1424–9. October 2014. doi:10.1016/j.rmed.2014.08.005. PMID 25256258. http://www.resmedjournal.com/article/S0954-6111(14)00287-X/pdf.
- ↑ "AdisInsight: Abediterol/mometasone". Springer International Publishing AG. http://adisinsight.springer.com/drugs/800033847.
 |
