Chemistry:Aminophylline
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a601015 |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous therapy (IV) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 60% |
| Elimination half-life | 7–9 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H24N10O4 |
| Molar mass | 420.434 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
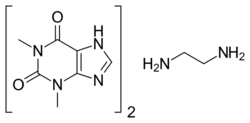
Aminophylline is a compound of the bronchodilator theophylline with ethylenediamine in 2:1 ratio. The ethylenediamine improves solubility, and the aminophylline is usually found as a dihydrate.[2]
Aminophylline is less potent and shorter-acting than theophylline. Its most common use is in the treatment of airway obstruction from asthma or COPD. Aminophylline is a nonselective adenosine receptor antagonist and phosphodiesterase inhibitor.[3]
Medical uses
Intravenous aminophylline can be used for acute exacerbation of symptoms and reversible airway obstruction in asthma and other chronic lung disease such as COPD, emphysema and chronic bronchitis. It is used as an adjunct to inhaled beta-2 selective agonists and systemically administered corticosteroids.[4]
Aminophylline is used to reverse regadenoson, dipyridamole or adenosine based infusions during nuclear cardiology stress testing. Aminophylline has also been reported to be effective in preventing slow heart rates during complex cardiovascular interventions (atherectomy of the right coronary artery).[5] It is also used in the treatment of heart block due to acute inferior myocardial infarction. It can also cause cardiac arrest.
Aminophylline has shown some promise as a bodyfat reducer when used as a topical cream.[6] Aminophylline is also a treatment option for anaphylactic shock.[7]
While it has been suggested for use in cardiac arrest evidence does not support a benefit.[8][9]
Side effects
Aminophylline can lead to theophylline toxicity. Aminophylline has been found to decrease the sedative effects of propofol[10] and decrease topiramate antiseizure action.[11]
Properties
It is more soluble in water than theophylline. White or slightly yellowish granules or powder, having a slight ammoniacal odor and a bitter taste. Upon exposure to air, it gradually loses ethylenediamine and absorbs carbon dioxide with the liberation of free theophylline. Its solutions are alkaline. 1 g dissolves in 25 mL of water to give a clear solution; 1 g dissolved in 5 mL of water crystallizes upon standing, but redissolves when a small amount of ethylenediamine is added. Insoluble in alcohol and in ether.
Pharmacology
Like other methylated xanthine derivatives, aminophylline is both a
- competitive nonselective phosphodiesterase inhibitor[12] which raises intracellular cAMP, activates PKA, inhibits TNF-alpha[13][14] and leukotriene[15] synthesis, and reduces inflammation and innate immunity[15] and
- nonselective adenosine receptor antagonist.[16]
Aminophylline causes bronchodilation, diuresis†, central nervous system and cardiac stimulation, and gastric acid secretion by blocking phosphodiesterase which increases tissue concentrations of cyclic adenosine monophosphate (cAMP) which in turn promotes catecholamine stimulation of lipolysis, glycogenolysis, and gluconeogenesis, and induces release of epinephrine from adrenal medulla cells.
†Note that diuresis is caused by an increase in cAMP which acts in the CNS to inhibit the release of antidiuretic hormone (arginine-vasopressin).
Adenosine is an endogenous extracellular messenger that can regulate myocardial oxygen needs.[3][17] It acts through cellular surface receptors which effect intracellular signalling pathways to increase coronary artery blood flow, slow heart rate, block atrioventricular node conduction, suppress cardiac automaticity, and decrease β-adrenergic effects on contractility.[3][17] Adenosine also antagonizes chronotropic and ionotropic effects of circulating catecholamines.[18] Overall, adenosine decreases the heart’s rate and force of contraction, which increases blood supply to the cardiac muscle. Given specific circumstances this mechanism (which is intended to protect the heart) may cause atropine-resistant refractory bradyasystole.[3] Adenosine's effects are concentration-dependent. Adenosine’s receptors are competitively antagonized by methylxanthines such as aminophylline.[3][17][18] Aminophylline competitively antagonizes the cardiac actions of adenosine at the cell surface receptors.[17] Thus, it increases heart rate and contractility.
Brand names
- Euphyllin
- Phyllocontin
- Truphylline
- Minomal R 175 mg tab
- Minomal R 350 mg tab
- Minomal SR 600 mg tab
References
- ↑ "FDA-Approved Drugs Abbreviated New Drug Application (ANDA): 087242". October 26, 1983. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=087242.
- ↑ "Aminophylline Professional Monograph". Drugs.com. https://www.drugs.com/pro/aminophylline-injection.html.
- ↑ 3.0 3.1 3.2 3.3 3.4 "A randomized controlled trial of intravenous aminophylline for atropine-resistant out-of-hospital asystolic cardiac arrest". Academic Emergency Medicine 10 (3): 192–197. March 2003. doi:10.1197/aemj.10.3.192. PMID 12615581.
- ↑ "Aminophylline Injection". https://www.drugs.com/pro/aminophylline-injection.html.
- ↑ "Aminophylline for Preventing Bradyarrhythmias During Orbital or Rotational Atherectomy of the Right Coronary Artery". https://www.invasivecardiology.com/articles/aminophylline-preventing-bradyarrhythmias-during-orbital-or-rotational-atherectomy-right.
- ↑ "Topical fat reduction from the waist". Diabetes, Obesity & Metabolism 9 (3): 300–303. May 2007. doi:10.1111/j.1463-1326.2006.00600.x. PMID 17391155.
- ↑ Blackbourne LH. Surgical Recall. Lippincott Williams and Wilkins, 2009. pp169
- ↑ "Aminophylline in bradyasystolic cardiac arrest". Emergency Medicine Journal 24 (8): 582–583. August 2007. doi:10.1136/emj.2007.051342. PMID 17652689.
- ↑ "Aminophylline for bradyasystolic cardiac arrest in adults". The Cochrane Database of Systematic Reviews 2015 (11): CD006781. November 2015. doi:10.1002/14651858.CD006781.pub3. PMID 26593309.
- ↑ "The effect of aminophylline on loss of consciousness, bispectral index, propofol requirement, and minimum alveolar concentration of desflurane in volunteers". Anesthesia and Analgesia 110 (2): 449–454. February 2010. doi:10.1213/ane.0b013e3181c6be7e. PMID 19955506.
- ↑ "Pharmacokinetic and pharmacodynamic interactions of aminophylline and topiramate in the mouse maximal electroshock-induced seizure model". European Journal of Pharmacology 562 (1–2): 53–59. May 2007. doi:10.1016/j.ejphar.2007.01.038. PMID 17320861.
- ↑ "Cyclic nucleotide phosphodiesterases". The Journal of Allergy and Clinical Immunology 108 (5): 671–680. November 2001. doi:10.1067/mai.2001.119555. PMID 11692087.
- ↑ "Insights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition". Clinics 63 (3): 321–328. June 2008. doi:10.1590/S1807-59322008000300006. PMID 18568240.
- ↑ "Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages". American Journal of Respiratory and Critical Care Medicine 159 (2): 508–511. February 1999. doi:10.1164/ajrccm.159.2.9804085. PMID 9927365.
- ↑ 15.0 15.1 "Leukotrienes: underappreciated mediators of innate immune responses". Journal of Immunology 174 (2): 589–594. January 2005. doi:10.4049/jimmunol.174.2.589. PMID 15634873.
- ↑ "Adenosine receptors: development of selective agonists and antagonists". Progress in Clinical and Biological Research 230 (1): 41–63. 1987. PMID 3588607.
- ↑ 17.0 17.1 17.2 17.3 "Adenosine receptor antagonism in refractory asystolic cardiac arrest: results of a human pilot study". Resuscitation 35 (1): 3–7. August 1997. doi:10.1016/s0300-9572(97)01097-6. PMID 9259053.
- ↑ 18.0 18.1 "Successful resuscitation using aminophylline in refractory cardiac arrest with asystole". Resuscitation 38 (1): 39–41. July 1998. doi:10.1016/s0300-9572(98)00079-3. PMID 9783508.
External links
- "Aminophylline". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/aminophylline.
 |

