Chemistry:Carmoterol
From HandWiki
Short description: Chemical compound
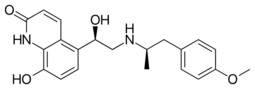 | |
| Clinical data | |
|---|---|
| Other names | TA-2005; CHF-4226 |
| Routes of administration | Inhalation |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H24N2O4 |
| Molar mass | 368.433 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Carmoterol (INN;[1] development codes TA-2005 and CHF-4226) is a non-catechol[2] experimental ultra-long-acting β adrenoreceptor agonist (ultra-LABA)[2][3] that was in clinical trials before 2010 when it has been withdrawn from further development based on evidence that the compound does not possess a competitive profile.[4]
Preliminary studies indicated duration of its effect exceeding 24 hours after inhalation of 3 μg.[2] The pharmacologic profile of this medication included the fact its potency in isolated guinea pig trachea is greater than that of formoterol and salmeterol. It is over 100 times more selective for bronchial muscle than myocardial tissue.[5]
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 53". WHO Drug Information 19 (1): 74. 2005. https://www.who.int/medicines/publications/druginformation/innlists/RL53.pdf. Retrieved 25 March 2016.
- ↑ 2.0 2.1 2.2 "27. Bronchodilators: Beta2-Agonists and Anticholingercs". Clinical Asthma. Philadelphia, PA: Mosby / Elsevier Health Sciences. 2008. ISBN 978-0-323-04289-5.
- ↑ "β(2) -adrenoceptor agonists: current and future direction". British Journal of Pharmacology 163 (1): 4–17. May 2011. doi:10.1111/j.1476-5381.2011.01216.x. PMID 21232045.
- ↑ "Chiesi: Annual Report 2010". Chiesi Farmaceutici S.p.A.. p. 20. http://www.chiesi.es/Assets/docs/Annual_report_2010.pdf.
- ↑ "New Options in COPD Therapy". Medscape. 2006. http://www.medscape.com/viewarticle/547182.
 |

