Chemistry:Umeclidinium bromide
From HandWiki
Short description: Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Incruse Ellipta |
| Other names | GSK573719A |
| License data | |
| Routes of administration | Inhalation (DPI) |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ~89%[2] |
| Metabolism | Liver (CYP2D6) |
| Elimination half-life | 11 hours |
| Excretion | Feces (58%) and urine (22%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
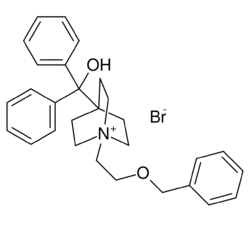
| Formula | C29H34BrNO2 |
| Molar mass | 508.500 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Umeclidinium bromide, sold under the brand name Incruse Ellipta, is a long-acting muscarinic antagonist approved for the maintenance treatment of chronic obstructive pulmonary disease (COPD).[2] It is also approved for this indication in combination with vilanterol (as umeclidinium bromide/vilanterol)[3][4] and also as a triple-therapy combination as fluticasone furoate/umeclidinium bromide/vilanterol.[5]
It is on the World Health Organization's List of Essential Medicines.[6] In 2020, it was the 245th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[7][8]
References
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2014". 21 June 2022. https://www.tga.gov.au/resources/resource/guidance/prescription-medicines-registration-new-chemical-entities-australia-2014.
- ↑ 2.0 2.1 "Incruse Ellipta (umeclidinium inhalation powder) for Oral Inhalation Use. Full Prescribing Information". GlaxoSmithKline, Research Triangle Park, NC 27709. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Incruse_Ellipta/pdf/INCRUSE-ELLIPTA-PI-PIL.PDF.
- ↑ "The combination of umeclidinium bromide and vilanterol in the management of chronic obstructive pulmonary disease: current evidence and future prospects". Therapeutic Advances in Respiratory Disease 7 (6): 311–9. December 2013. doi:10.1177/1753465813499789. PMID 24004659.
- ↑ "FDA Approves Umeclidinium and Vilanterol Combo for COPD". Medscape. December 18, 2013. http://www.medscape.com/viewarticle/817964.
- ↑ "TRELEGY ELLIPTA Package Insert". GlaxoSmithKline. September 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209482s000lbl.pdf.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Umeclidinium - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Umeclidinium.
External links
- "Umeclidinium". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/umeclidinium.
- "Umeclidinium bromide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/umeclidinium%20bromide.
 |

