Chemistry:Flotufolastat F-18
From HandWiki
Short description: Medication
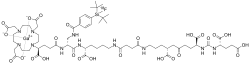 Flotufolastat F-18 gallium | |
| Clinical data | |
|---|---|
| Trade names | Posluma |
| Other names | 18F-rhPSMA-7.3 |
| License data | |
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| 3D model (JSmol) | |
| |
| |
Flotufolastat F-18, sold under the brand name Posluma, is a radioactive diagnostic agent for use with positron emission tomography (PET) imaging for prostate cancer.[1] The active ingredient is flotufolastat F-18 gallium.[1]
Flotufolastat F-18 was approved for medical use in the United States in May 2023.[1][2]
Medical uses
Flotufolastat F-18 is indicated for positron emission tomography of prostate-specific membrane antigen positive lesions in men with prostate cancer.[1][3]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Posluma- flotufolastat f-18 injection". 2 June 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=907ac4a4-a088-4826-ab50-cfc31985c9d4.
- ↑ "U.S. FDA Approves Blue Earth Diagnostics' Posluma (Flotufolastat F 18) Injection, First Radiohybrid PSMA-targeted PET Imaging Agent for Prostate Cancer" (Press release). Blue Earth Therapeutics. 30 May 2023. Retrieved 25 June 2023 – via Business Wire.
- ↑ "Flotufolastat F 18: Diagnostic First Approval". Molecular Diagnosis & Therapy 27 (5): 631–636. September 2023. doi:10.1007/s40291-023-00665-y. PMID 37439946. https://figshare.com/articles/online_resource/Flotufolastat_F_18_Diagnostic_First_Approval/23631417.
External links
- Clinical trial number NCT04186819 for "Imaging Study to Investigate the Safety and Diagnostic Performance of rhPSMA 7.3 (18F) in Newly Diagnosed Prostate Cancer (LIGHTHOUSE)" at ClinicalTrials.gov
- Clinical trial number NCT04186845 for "Imaging Study to Investigate Safety and Diagnostic Performance of rhPSMA 7.3 (18F) PET Ligand in Suspected Prostate Cancer Recurrence (SPOTLIGHT)" at ClinicalTrials.gov
 |

