Biology:Fluciclovine (18F)
 | |
| Clinical data | |
|---|---|
| Trade names | Axumin |
| License data |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
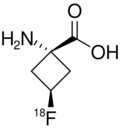
| Formula | C5H818FNO2 |
| Molar mass | 132.12 g/mol |
Fluciclovine (18F), also known as anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (anti-3[18F] FACBC),[1][2] or as Axumin (brand name), is a diagnostic agent indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated prostate specific antigen (PSA) levels.[3][4]
Background
Most imaging tests have not been able to localize recurrent prostate cancer when the PSA is mildly increased.[1][3] Axumin scans were compared to [11C]-tagged choline PET scans, another FDA approved PET scan that can assist in this situation, and to biopsy results.[3][5] Fluciclovine tagged PET scans appear to more sensitive than CT scans[6] and to [11C]-tagged choline PET scans.[7][8]
Mechanism
Fluciclovine is a [18F]-tagged synthetic analog of the amino acid L-leucine.[9][10] FACBC uptake by the tumor is related to functional activity of two amino acid transporters,[11] specifically sodium-dependent system ASC, with a lesser contribution by sodium-independent system L.[10] Although it is handled by the amino acid transporter system, it does not undergo terminally incorporative metabolism within the body.[10] The distribution of the tracer in the body differs from choline and FDG, as kidney uptake of FACBC is negligible, and no activity is found in the urinary tract.[10][11] There is low native brain uptake compared to FDG, which may enhance detection of brain metastases[2][10] or primary brain tumors.[10] The more intense native liver and pancreatic uptake seen with this agent would be expected to limit disease detection in those organs.[10] FACBC has a short synthesis time and a long half-life, which eliminate the need for an onsite cyclotron.[11]
Marketing
Axumin is marketed by Blue Earth Diagnostics, Ltd., United Kingdom.[4]
References
- ↑ 1.0 1.1 "The dilemma of localizing disease relapse after radical treatment for prostate cancer: which is the value of the actual imaging techniques?". Curr Radiopharm 6 (2): 92–5. 2013. doi:10.2174/1874471011306020005. PMID 23597246.
- ↑ 2.0 2.1 Forrest W. "Start-up develops prostate PET agent." AuntMinnie.com May 9, 2014 [1]
- ↑ 3.0 3.1 3.2 FDA Press Release. "FDA approves new diagnostic imaging agent to detect recurrent prostate cancer" May 27, 2016 [2]
- ↑ 4.0 4.1 Drugs.com "FDA Approves Axumin (fluciclovine F 18) Diagnostic Imaging Agent to Detect Recurrent Prostate Cancer" May 27, 2016 [3]
- ↑ Berberabe T. "FDA Approves Radioactive Imaging Agent Axumin in Recurrent Prostate Cancer." OncLive May 27, 2016 [4]
- ↑ "Recurrent prostate cancer detection with anti-3-[(18)FFACBC PET/CT: comparison with CT"]. Eur. J. Nucl. Med. Mol. Imaging 43 (10): 1773–1783. 2016. doi:10.1007/s00259-016-3383-8. PMID 27091135.
- ↑ "New Clinical Indications for (18)F/(11)C-choline, New Tracers for Positron Emission Tomography and a Promising Hybrid Device for Prostate Cancer Staging: A Systematic Review of the Literature". Eur. Urol. 70 (1): 161–75. 2016. doi:10.1016/j.eururo.2016.01.029. PMID 26850970.
- ↑ "18F-Fluciclovine PET/CT for the Detection of Prostate Cancer Relapse: A Comparison to 11C-Choline PET/CT". Clin Nucl Med 40 (8): e386–91. 2015. doi:10.1097/RLU.0000000000000849. PMID 26053708.
- ↑ Bankhead C. "Prostate Scan Agent Approved by FDA." MedPage Today May 27, 2016 [5]
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 "Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease". J. Nucl. Med. 55 (12): 1986–92. 2014. doi:10.2967/jnumed.114.143628. PMID 25453047.
- ↑ 11.0 11.1 11.2 "The new promise of FACBC position emission tomography/computed tomography in the localization of disease relapse after radical treatment for prostate cancer: are we turning to the right radiotracer?". Eur. Urol. 65 (1): 255–6. 2014. doi:10.1016/j.eururo.2013.08.053. PMID 24094575.
External links
- "Fluciclovine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/fluciclovine.
 |

