Chemistry:GTPgammaS
| |
| Names | |
|---|---|
| IUPAC name
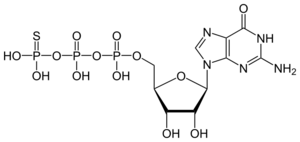
[(2S,3R,4S,5S)-5-(2-amino-6-oxo-3H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydroxyphosphinothioyl hydrogen phosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C10H16N5O13P3S | |
| Molar mass | 539.24 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
GTPgammaS (GTPγS, guanosine 5'-O-[gamma-thio]triphosphate) is a non-hydrolyzable or slowly hydrolyzable G-protein-activating analog of guanosine triphosphate (GTP). Many GTP binding proteins demonstrate activity when bound to GTP, and are inactivated via the hydrolysis of the phosphoanhydride bond that links the γ-phosphate to the remainder of the nucleotide, leaving a bound guanosine diphosphate (GDP) and releasing an inorganic phosphate. This usually occurs rapidly, and the GTP-binding protein can then only be activated by exchanging the GDP for a new GTP molecule.[1] The substitution of sulfur for one of the oxygens of the γ-phosphate of GTP creates a nucleotide that either cannot be hydrolyzed or is only slowly hydrolyzed. This prevents the GTP-binding proteins from being inactivated, and allows the cellular processes that they carry out when active to be more easily studied.[2]
The consequences of the constitutive activation of GTP-binding proteins include stimulation of phosphoinositide hydrolysis,[3] cyclic AMP accumulation or elimination,[4] and activation of specific proto-oncogenes.[5] The 35S labelled radioligand of the compound, 35SGTPγS, is used in autoradiography and G-protein binding studies.[6]
References
- ↑ Harrison, C.; Traynor, J. R. (December 12, 2003). "The [35SGTPγS binding assay: approaches and applications in pharmacology"]. Life Sciences 74 (4): 489–508. doi:10.1016/j.lfs.2003.07.005. PMID 14609727. https://www.sciencedirect.com/science/article/abs/pii/S0024320503008920. Retrieved April 26, 2021.
- ↑ Spoerner, Michael; Nuehs, Andrea; Herrmann, Christian; Steiner, Guido; Kalbitzer, Hans Robert (March 2007). "Slow conformational dynamics of the guanine nucleotide‐binding protein Ras complexed with the GTP analogue GTPγS". The FEBS Journal 274 (6): 1419–1433. doi:10.1111/j.1742-4658.2007.05681.x. PMID 17302736.
- ↑ White, H. L.; Scates, P. W. (1991). "Effects of GTPγS and other nucleotides on phosphoinositide metabolism in crude rat brain synaptosomal preparations". Neurochemistry International 18 (3): 381–387. doi:10.1016/0197-0186(91)90170-I. PMID 20504715. https://www.sciencedirect.com/science/article/abs/pii/019701869190170I. Retrieved April 26, 2021.
- ↑ Baker, Stephen P.; Scammells, Peter J.; Belardinelli, Luiz (July 2000). "Differential A1-adenosine receptor reserve for inhibition of cyclic AMP accumulation and G-protein activation in DDT1 MF-2 cells". British Journal of Pharmacology 130 (5): 1156–1164. doi:10.1038/sj.bjp.0703405. PMID 10882402.
- ↑ Pennington, Stephen R.; Hesketh, T. Robin; Metcalfe, James C. (January 25, 1988). "GTPγS activation of proto-oncogene expression in transiently permeabilised Swiss 3T3 fibroblasts". FEBS Letters 227 (2): 203–208. doi:10.1016/0014-5793(88)80899-8. PMID 3276558.
- ↑ Strange, Philip G. (November 2010). "Use of the GTPγS ([35SGTPγS and Eu-GTPγS) binding assay for analysis of ligand potency and efficacy at G protein-coupled receptors"]. British Journal of Pharmacology 161 (6): 1238–1249. doi:10.1111/j.1476-5381.2010.00963.x. PMID 20662841.
