Chemistry:Gallium (68Ga) gozetotide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Illuccix, Locametz |
| Other names | Gallium 68 PSMA-11, Gallium Ga 68 gozetotide (USAN US) |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Excretion | Urine[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| 3D model (JSmol) | |
| |
| |
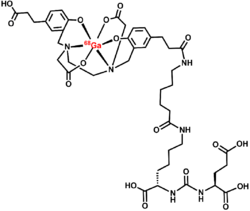
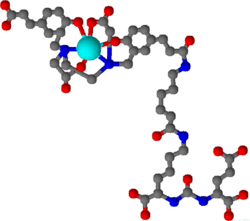
Gallium (68Ga) gozetotide or Gallium (68Ga) PSMA-11 sold under the brand name Illuccix among others, is a radiopharmaceutical made of 68Ga conjugated to prostate-specific membrane antigen (PSMA) targeting ligand, Glu-Urea-Lys(Ahx)-HBED-CC, used for imaging prostate cancer by positron emission tomography (PET).[10] The PSMA targeting ligand specifically directs the radiolabeled imaging agent towards the prostate cancerous lesions in men.[11]
The most common side effects with gallium (68Ga)-radiolabelled gozetotide are tiredness, nausea (feeling sick), constipation and vomiting.[9]
Gallium (68Ga) gozetotide was approved for medical use in the United States in December 2021,[12][13] and in the European Union in December 2022.[9] It is the first drug approved by the US Food and Drug Administration (FDA) as a PET imaging agent.[11]
Structure
Radiopharmaceuticals based on HBED are composed of three components: a chelator that has a HBED structure and two functions, a radiometal coordinated with the chelator, and a binding motif or pharmacophore that is conjugated to the chelator (such as a peptide or antibody). One of the most popular HBED chelators is HBED-CC. This chelator can create stable complexes with trivalent gallium at normal temperatures and it attaches to bioactive molecules through its propionic acid moieties.[14]
Medical uses
Gallium (68Ga) gozetotide is a radioactive diagnostic agent indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA)-positive lesions in men with prostate cancer.[7][8][9]
Ga 68 PSMA-11 injections are used for PET imaging of prostate-specific membrane antigen (PSMA) positive lesions in males with prostate cancer. It can be given for the patients with suspected metastasis, and the candidates with initial definitive therapy.[2]
History
In the early 2000s, researchers began exploring the use of PSMA as a target for imaging and therapy. The first PSMA-targeted radiotracer was developed using a different radioactive element, technetium-99m. This radiotracer, called 99mTc-MIP-1404, showed promise in preclinical studies but did not perform well in clinical trials.[15]
In 2011, researchers started investigating the use of gallium-68, a different radioactive element, as a more suitable alternative for PSMA-targeted radiotracers. In 2013, the first Ga-PSMA radiotracer was developed by researchers at DKFZ in Germany, and it showed promising results in early clinical studies.[16]
Since then, Ga-PSMA has been extensively studied in clinical trials, and it has been found to be a highly effective imaging agent for detecting prostate cancer lesions. It is now widely used in clinical practice, particularly for patients with recurrent prostate cancer and those with high-risk disease.
Initially gallium (68Ga) chloride solution injections used for radiolabelling,[17] in 2019 European Pharmacopoeia mentions gallium (68Ga) DOTATOC injection for radiolabelling and PET imaging.[18]
Ga 68 PSMA-11 is co-developed by University of California, Los Angeles and University of California, San Francisco, they conducted phase III clinical trial.[19] In December 2020, the drug was first approved by US Food and Drug Administration (FDA) for PET imaging.[11]
Mechanism of action
Gallium (68Ga) gozetotide binds with prostate-specific membrane antigen (PSMA).[2] This binds to cells that express PSMA, including malignant prostate cancer cells.[2] The radioactive isotope of gallium, 68Ga is responsible for emitting β+ radiations and X-rays.[2] This helps in recording images by positron emission tomography (PET) and CT scan.[2]
Society and culture
Legal status
On 13 October 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Locametz, intended for the diagnosis of prostate cancer.[20] The applicant for this medicinal product is Novartis Europharm Limited.[20] Locametz was approved for medical use in the European Union in December 2022.[9][21]
Names
Gallium (68Ga) gozetotide is the international nonproprietary name (INN).[22]
References
- ↑ 1.0 1.1 "AusPAR: Glu-urea-Lys(ahx)-hbed-CC". 27 June 2022. https://www.tga.gov.au/auspar/auspar-glu-urea-lysahx-hbed-cc.[yes|permanent dead link|dead link}}]
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Gallium GA-68 PSMA-11- gallium ga-68 gozetotide injection, solution". 21 November 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7744bafb-755e-29a2-e053-2991aa0a86a9.
- ↑ "Illuccix Summary Basis of Decision". https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?lang=en&linkID=SBD00624&lang=en.
- ↑ "Summary Basis of Decision for Locametz". 21 June 2023. https://dhpp.hpfb-dgpsa.ca/review-documents/resource/SBD1687370834165.
- ↑ "Details for: Locametz". 11 May 2023. https://dhpp.hpfb-dgpsa.ca/dhpp/resource/102582.
- ↑ "Gallium GA-68 PSMA-11- gallium ga-68 gozetotide injection, solution". 30 November 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b6253a7b-0f73-36be-e053-2a95a90a9015.
- ↑ 7.0 7.1 "Illuccix- kit for the preparation of gallium ga 68 gozetotide injection kit". 12 May 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d4643b31-9b4f-673f-e053-2a95a90a559d.
- ↑ 8.0 8.1 "Locametz- kit for the preparation of gallium ga 68 gozetotide injection, powder, lyophilized, for solution". 23 March 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1ea62b65-8138-4a88-8a33-4060ececa42f.
- ↑ 9.0 9.1 9.2 9.3 9.4 "Locametz EPAR". 12 October 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/locametz. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "gallium Ga 68 gozetotide". 2 February 2011. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/gallium-ga-68-gozetotide?redirect=true.
- ↑ 11.0 11.1 11.2 "FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer" (Press release). U.S. Food and Drug Administration (FDA). 2 December 2020. Archived from the original on 4 November 2021. Retrieved 9 November 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Drug Approval Package: Illuccix". U.S. Food and Drug Administration (FDA). 3 August 2022. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/214032Orig1s000TOC.cfm.
- ↑ "Drug Approval Package: Locametz". U.S. Food and Drug Administration (FDA). 2 June 2022. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/215841Orig1s000TOC.cfm.
- ↑ "A Multifunctional HBED-Type Chelator with Dual Conjugation Capabilities for Radiopharmaceutical Development". Synlett 30 (15): 1795–1798. 2019. doi:10.1055/s-0039-1690194.
- ↑ "Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer". J Nucl Med 53 (12): 1883–1891. 2012. doi:10.2967/jnumed.112.105361. PMID 23132604.
- ↑ "PET/MRI with a 68Ga-PSMA ligand for the detection of prostate cancer". Eur J Nucl Med Mol Imaging 40 (10): 1629–1630. 2013. doi:10.1007/s00259-013-2489-5. PMID 23817686.
- ↑ "Gallium (68Ga) Chloride Solution for Radiolabelling". European Pharmacopoeia (9th ed.). Stuttgart. 2018. p. 1148. ISBN 978-3-7692-6816-4.
- ↑ "Gallium (68Ga) DOTATOC injection". European Pharmacopoeia (10th ed.). Stuttgart. 2019. p. 1208. ISBN 978-3-7692-7453-0.
- ↑ "68Ga-PSMA-11 NDA Approval: A Novel and Successful Academic Partnership". Journal of Nuclear Medicine 62 (2): 149–155. February 2021. doi:10.2967/jnumed.120.260455. PMID 33443068.
- ↑ 20.0 20.1 "Locametz: Pending EC decision". 13 October 2022. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/locametz. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Locametz Product information". https://ec.europa.eu/health/documents/community-register/html/h1692.htm.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 85". WHO Drug Information 35 (1). 2021. https://cdn.who.int/media/docs/default-source/international-nonproprietary-names-(inn)/rl85.pdf. Retrieved 28 May 2022.
External links
- "Gallium Ga 68 gozetotide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/gallium%20ga%2068%20gozetotide.
 |

