Chemistry:Germanium monoselenide
| |

| |
| Names | |
|---|---|
| IUPAC name
Germanium selenide
| |
| Other names
germanium(II) selenide
| |
| Identifiers | |
PubChem CID
|
|
| Properties | |
| GeSe | |
| Molar mass | 151.57 g/mol |
| Appearance | black |
| Density | 5.56 g/cm3 |
| Melting point | 667 °C (1,233 °F; 940 K) (decomposes) |
| Band gap | 1.33 eV (direct) [1] |
Refractive index (nD)
|
2.5 |
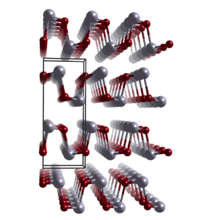
| Structure | |
| Orthorhombic | |
| Pnma | |
| Related compounds[2] | |
Other anions
|
Germanium monoxide Germanium monosulfide Germanium telluride |
Other cations
|
Tin selenide Lead selenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Germanium monoselenide is a chemical compound with the formula GeSe. It exists as black crystalline powder having orthorhombic (distorted NaCl-type) crystal symmetry; at temperatures ~650 °C, it transforms into the cubic NaCl structure.[3] GeSe has been shown to have stereochemically active Ge 4s lone pairs that are responsible for the distorted structure and the relatively high position of the valence band maximum with respect to the vacuum level.[4]
To grow GeSe crystals, GeSe powder is vaporized at the hot end of a sealed ampule and allowed to condense at the cold end. Usual crystals are small and show signs of irregular growth, caused mainly by convective motion in the gaseous medium. However, GeSe grown under condition of zero-gravity and reduced convection aboard the Skylab are ~10 times larger than Earth-grown crystals, and are free from visual defects.[5][6]
References
- ↑ Philip A. E. Murgatroyd, Matthew J. Smiles, Christopher N. Savory, Thomas P. Shalvey, Jack E. N. Swallow, Nicole Fleck, Craig M. Robertson, Frank Jäckel, Jonathan Alaria, Jonathan D. Major, David O. Scanlon, and Tim D. Veal (2020). "GeSe: Optical Spectroscopy and Theoretical Study of a van der Waals Solar Absorber". Chemistry of Materials 32 (7): 3245–3253. doi:10.1021/acs.chemmater.0c00453. PMID 32308255.
- ↑ GeSe at webelements
- ↑ Wiedemeier H., Siemers P.A. (1975). "The Thermal Expansion and High Temperature Transformation of GeSe". Zeitschrift für anorganische und allgemeine Chemie 411: 90–96. doi:10.1002/zaac.19754110110.
- ↑ M. J. Smiles, J. M. Skelton, H. Shiel, L. A. H. Jones, J. E. N. Swallow, H. J. Edwards, T. J. Featherstone, P. A. E. Murgatroyd, P. K. Thakur, Tien-Lin Lee, V. R. Dhanak, and T. D. Veal (2021). "Ge 4s2 Lone Pairs and Band Alignments in GeS and GeSe for photovoltaics". J. Mater. Chem. A 9 (39): 22440–22452. doi:10.1039/D1TA05955F.
- ↑ "SP-400 Skylab, Our First Space Station". NASA. https://history.nasa.gov/SP-400/ch10.htm.
- ↑ H. Wiedemeier (1975). "Crystal growth and transport rates of GeSe and GeTe in micro-gravity environment". Journal of Crystal Growth 31: 36. doi:10.1016/0022-0248(75)90107-4. Bibcode: 1975JCrGr..31...36W.
 |

