Chemistry:Germanium tetrafluoride
| |
| Names | |
|---|---|
| IUPAC names
Germanium tetrafluoride
Tetrafluorogermane Tetrafluoridogermanium | |
| Other names
Germanium(IV) fluoride
Germanium fluoride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties[2] | |
| GeF4 | |
| Molar mass | 148.634 g/mol |
| Appearance | colourless gas |
| Density | 6.074 g/L (gas), 2.46 g/mL (liquid)[1] |
| Melting point | −15 °C (5 °F; 258 K) at 4 bar |
| Boiling point | −36.5 °C (−33.7 °F; 236.7 K) sublimates |
| −50.0·10−6 cm3/mol | |
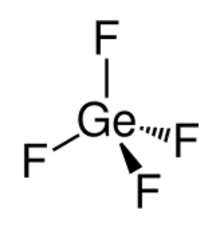
| Structure | |
| tetrahedral | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-8.008 kJ/g |
| Hazards | |
| Main hazards | Reacts with water to form HF, corrosive |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H280, H314, H331, H372 | |
| P260, P261, P264, P270, P271, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P311, P314, P321, P363, P403+233, P405, P410+403, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Germanium tetrachloride Germanium tetrabromide Germanium tetraiodide |
Other cations
|
Carbon tetrafluoride Silicon tetrafluoride Tin tetrafluoride Lead tetrafluoride |
Related compounds
|
Germanium difluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Germanium tetrafluoride (GeF4) is a chemical compound of germanium and fluorine. It is a colorless gas.
Synthesis
Germanium tetrafluoride is formed by treating germanium with fluorine:
- Ge + 2 F2 → GeF4
Alternatively germanium dioxide combines with hydrofluoric acid (HF):[3]
- GeO2 + 4 HF → GeF4 + 2 H2O
It is also formed during the thermal decomposition of a complex salt, Ba[GeF6]:[4]
- Ba(GeF6) → GeF4 + BaF2
Properties
Germanium tetrafluoride is a noncombustible, strongly fuming gas with a garlic-like odor. It reacts with water to form hydrofluoric acid and germanium dioxide. Decomposition occurs above 1000 °C.[5]
Reaction of GeF4 with fluoride sources produces GeF5− anions with octahedral coordination around Ge atom due to polymerization.[6] The structural characterization of a discrete trigonal bipyramidal GeF5− anion was achieved by a "naked" fluoride reagent 1,3-bis(2,6-diisopropylphenyl)imidazolium fluoride.[7]
Uses
In combination with disilane, germanium tetrafluoride is used for in the synthesis of SiGe.[1]
References
- ↑ 1.0 1.1 Germanium(IV) fluoride. sigmaaldrich.com
- ↑ Lide, D. R., ed (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 4.64. ISBN 0-8493-0486-5.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 376–377. ISBN 978-0-08-037941-8.
- ↑ Georg Brauer: Handbuch der Präparativen Anorganischen Chemie
- ↑ Germaniumtetrafluorid. IFA Database
- ↑ Mallouk, T. E.; Desbat, B.; Bartlett, N. (1984). "Structural Studies of salts of cis and trans μ-Fluoro-Bridged Polymers of Pentafluorogermanate(1-) and of the Pentafluorogermanate(1-) Monomer". Inorganic Chemistry 23 (20): 3160-3166. doi:10.1021/ic00188a027.
- ↑ Alič, B.; Tramšek, M.; Kokalj, A.; Tavčar, G. (2017). "Discrete GeF5– Anion Structurally Characterized with a Readily Synthesized Imidazolium Based Naked Fluoride Reagent". Inorganic Chemistry 56 (16): 10070–10077. doi:10.1021/acs.inorgchem.7b01606. PMID 28792216.
External links
 |


