Chemistry:Germanium difluoride
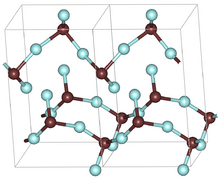 Two unit cells of the orthorhombic GeF2 structure. Brown atoms are germanium and cyan atoms are fluorine
| |
| Names | |
|---|---|
| IUPAC names
Germanium difluoride
Difluorogermylidene Difluoridogermanium | |
| Other names
Germanium(II) fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties[1] | |
| GeF2 | |
| Molar mass | 110.61 g/mol |
| Appearance | White orthorhombic hygroscopic crystals |
| Density | 3.61 g/cm3 |
| Melting point | 110 °C (230 °F; 383 K) |
| Boiling point | 130 °C (266 °F; 403 K) (sublimates) |
| Hazards | |
| Main hazards | Reacts with water to form HF, corrosive |
| Related compounds | |
Related compounds
|
Germanium tetrafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Germanium difluoride (GeF2) is a chemical compound of germanium and fluorine. It is a white solid with a melting point of 110 °C, and can be produced by reacting germanium tetrafluoride with germanium powder at 150–300 °C.[2]
Structure
Germanium difluoride forms orthorhombic crystals with a space group P212121 (No. 19), Pearson symbol oP12, and lattice constants a = 0.4682 nm, b = 0.5178 nm, c = 0.8312 nm, Z = 4 (four structure units per unit cell). Its crystal structure is characterized by strong polymeric chains composed by GeF3 pyramids. One of the fluorine atom in the pyramid is shared by two neighboring chains, providing a weak link between them.[3] Another, less common crystal form of GeF2 has tetragonal symmetry with a space group P41212 (No. 92), Pearson symbol tP12, and lattice constants a = 0.487 nm, b = 0.6963 nm, c = 0.858 nm.[4]
References
- ↑ Lide, D. R., ed (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 4.64. ISBN 0-8493-0486-5.
- ↑ Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (second ed.). Butterworth Heinemann. pp. 376–377. ISBN 0-7506-3365-4.
- ↑ Trotter, James; Akhtar, M.; Bartlett, Neil (1966). "The crystal structure of germanium difluoride". Journal of the Chemical Society A: Inorganic, Physical, Theoretical: 30. doi:10.1039/J19660000030.
- ↑ G.P. Adams; L.M. Albritton; D.W. Bonnell; J.L. Margrave; J. Scott; P.W. Wilson (1971). "A new solid phase in germanium difluoride". Journal of the Less Common Metals 24 (1): 113–116. doi:10.1016/0022-5088(71)90174-3.
 |

