Chemistry:Gliflozin
Gliflozin drugs are a class of medications that inhibit reabsorption of glucose in the kidney and therefore lower blood sugar.[1] They act by inhibiting sodium-glucose transport protein 2 (SGLT2), and are therefore also called SGLT2 inhibitors. Gliflozins are used in the treatment of type II diabetes mellitus (T2DM). Apart from glycemic control, gliflozins have been shown to provide significant cardiovascular benefit in T2DM patients.[2] Several drugs of this class have been approved or are currently under development.[3] In studies on canagliflozin, a member of this class, the drug was found to enhance blood sugar control as well as reduce body weight and systolic and diastolic blood pressure.[4]
Medical uses
The gliflozins are used to treat type 2 diabetes mellitus but are most often used as second- or third-line agents instead of first-line because there are other drugs on the market that have much longer safety record and are less expensive than gliflozins.[5]
Gliflozins may be a good option for patients who are failing with metformin monotherapy, especially if weight is part of the underlying treatment.[6] They are often used in combination therapy, for example the dual therapy metformin plus gliflozin and the triple therapy metformin, sulphonylurea and gliflozin.[5]
A recent systematic review and network meta-analysis (comparing SGLT-2 inhibitors, GLP-1 agonists and DPP-4 inhibitors) demonstrated that use of SGLT2 inhibitors was associated with a 20% reduction in death compared with placebo or no treatment.[7]
Adverse effects
Genital infections seem to be the most common adverse effect of gliflozins. In clinical trials mycotic infections, urinary tract infections and osmotic diuresis were higher in patients treated with gliflozins.
In May 2015 FDA issued a warning that gliflozins can increase risk of diabetic ketoacidosis (DKA).[8] By reducing glucose blood circulation, gliflozins cause less stimulation of endogenous insulin secretion or lower dose of exogenous insulin that results in diabetic ketoacidosis (DKA). They can also contribute euglycemic DKA (euDKA) because of the renal tubular absorption of ketone bodies.[9]
In September 2015 FDA issued a warning related to canagliflozin (Invokana®) and canagliflozin/metformin (Invokamet®) due to decreased bone mineral density and therefore increased risk of bone fractures. Using gliflozins in combination therapy with metformin can lower the risk of hypoglycemia compared to other T2BM such as sulfonylureas and insulin.[8]
Increased risk of lower limb amputation is associated with canagliflozin but further data is needed to confirm this risk associated with different gliflozins.[10]
Interactions
Interactions are important for SGLT2 inhibitors because most T2DM patients are taking many other medications. Gliflozins can increase the diuretic effect of thiazides, loop diuretics and related diuretics and therefore increase the risk of dehydration and hypotension.[11] It is important to adjust the dose of antidiabetics if the treatment is combination therapy to avoid hypoglycemia. For example interactions with sulfonylureas have lead to severe hypoglycemia presumably due to cytochrome P450.[12]
A study has shown that it is safe to consume dapagliflozin along with pioglitazone, metformin, glimepiride, or sitagliptin and dose adjustment is unnecessary for either drug.[13] It is unlikely that food intake has clinical meaningful impact on the efficacy of dapagliflozin, therefore it can be administered without regard to meals.[13][14]
Members
These are the known members of the gliflozin class:
- Canagliflozin was the first SGLT2 inhibitor to be approved for use in the United States. It was approved in March 2013 under the brand name Invokana and it was also marketed throughout the EU under the same name.[15][16]
- Dapagliflozin is the first SGLT2 inhibitor approved anywhere in the world, it happened in 2011 by the EU. It was approved for use in the United States under the brand name Farxiga by the Food and Drug Administration in January 2014.[17]
- Empagliflozin, approved in the United States in August 2014 under the brand name Jardiance by Boehringer Ingelheim.[18] Of the gliflozins, empagliflozin and tofogliflozin have the highest specificity for SGLT2 inhibition.[1] It is the only oral medicine for type 2 diabetes that has been shown to reduce the risk of cardiovascular death.[19]
- Ertugliflozin was approved in the United States under the brand name Steglatro in December 2017.[20]
- Ipragliflozin, produced by the Japanese company Astellas Pharma Inc. under the brand name Suglat, approved in Japan January 2014.[21][22]
- Luseogliflozin was approved in Japan March 2014 under the brand name Lusefi and was developed by Taisho Pharmaceutical.[23]
- Remogliflozin etabonate discontinued in clinical trials.[24]
- Sergliflozin etabonate discontinued after Phase II trials.[24]
- Sotagliflozin is a dual SGLT1/SGLT2 inhibitor in phase III trials under the brand name Zynquista. Developed by Lexicon pharmaceuticals. If approved, sotagliflozin would be the first oral treatment in combination with insulin to treat type 1 diabetes mellitus.[25]
- Tofogliflozin was approved in Japan March 2014 under the brand names Apleway and Deberza developed by Sanofi and Kowa Pharmaceutical[26]
Mechanism of action
Sodium Glucose cotransporters (SGLTs) are proteins that occur primarily in the kidneys and play an important role in maintaining glucose balance in the blood.[27] SGLT1 and SGLT2 are the two most know SGLTs of this family. SGLT2 is the major transport protein and promotes reabsorption from the glomerular filtration glucose back into circulation and is responsible for approximately 90% of the kidney's glucose reabsorption.[1] SGLT2 is mainly expressed in the kidneys on the epithelial cells lining the first segment of the proximal convoluted tubule. By inhibiting SGLT2, gliflozins prevent the kidneys' reuptake of glucose from the glomerular filtrate and subsequently lower the glucose level in the blood and promote the excretion of glucose in the urine (glucosuria).[28][29]
The mechanism of action on a cellular level is not well understood. However, it has been shown that binding of different sugars to the glucose site affects the orientation of the aglycone in the access vestibule. So when the aglycone binds it affects the entire inhibitor. Together these mechanisms lead to a synergistic interaction. Therefore, variations in the structure of both the sugar and the aglycone are crucial for the pharmacophore of SGLT inhibitors.[30]
Dapagliflozin is an example of an SGLT-2 inhibitor, it is a competitive, highly selective inhibitor of SGLT. It acts via selective and potent inhibition of SGLT-2, and its activity is based on each patient’s underlying blood sugar control and kidney function. The results are decreased kidney reabsorption of glucose, glucosuria effect increases with higher level of glucose in the blood circulation. Therefore, dapagliflozin reduces the blood glucose concentration with a mechanism that is independent of insulin secretion and sensitivity, unlike many other antidiabetic drugs. Functional pancreatic β-cells are not necessary for the activity of the drug so it is convenient for patients with diminished β-cell function.[28][29]
Sodium and glucose are co-transported by the SGLT-2 protein into the tubular epithelial cells across the brush-border membrane of the proximal convoluted tubule. This happens because of the sodium gradient between the tubule and the cell and therefore provides a secondary active transport of glucose. Glucose is later reabsorbed by passive transfer of endothelial cells into the interstitial glucose transporter protein.[28][29][31]
| SGLT | Expressed in human tissues |
|---|---|
| SGLT1 | Intestine, trachea, kidney, heart, brain, testis, prostate |
| SGLT2 | Kidney, brain, liver, thyroid, muscle, heart |
Pharmacology
The elimination half-life, bioavailability, protein binding, the blood concentration Cmax at time tmax, and other pharmacokinetic parameters of various drugs of this class are present in table 2. These drugs are excreted in the urine as inactive metabolites.[31][32][33][34]
| Name of drug | Bioavailability | Protein binding | tmax (hours) | t1/2 (hours) | Cmax | SGLT2 selectivity over SGLT1 |
|---|---|---|---|---|---|---|
| Canagliflozin | 65% (300 mg dose) | 99% | 1–2 | 10.6 (100 mg dose); 13.1 (300 mg dose) | 1096 ng/mL (100 mg dose); 3480 ng/mL (300 mg dose) | 250 fold |
| Dapagliflozin | 78% | 91% | 1–1.5 | 12.9 | 79.6 ng/mL (5 mg dose); 165.0 ng/mL (10 mg dose) | 1200 fold |
| Empagliflozin | 90–97% (mice); 89% (dogs); 31% (rats) | 86.20% | 1.5 | 13.2 (10 mg dose); 13.3h (25 mg dose) | 259nmol/L (10 mg dose); 687nmol/L (25 mg dose) | 2500 fold |
| Ertugliflozin | 70-90% | 95% | 0.5-1.5 | 11-17 | 268 ng/mL (15 mg dose) | 2000 fold |
| Ipragliflozin (50 mg) | 90% | 96.30% | 1 | 15–16 (50 mg dose) | 975 ng/mL | 360 fold |
| Luseogliflozin | 35.3% (male rats); 58.2% (female rats); 92.7% (male dogs) | 96.0–96.3% | 0.625±0.354 | 9.24±0.928 | 119±27.0 ng/mL | 1650 fold |
| Tofogliflozin (20 mg) | 97.50% | 83% | 0.75 | 6.8 | 489 ng/mL | 2900 fold |
- Cmax: Maximum serum concentration that drug achieves in body after the drug has been and administrated
- tmax: Time to achieve maximum plasma concentration
- t1/2: Biological half-life
In studies that were made on healthy people and people with type II diabetes mellitus, who were given dapagliflozin in either single ascending dose (SAD) or multiple ascending dose (MAD) showed results that confirmed a pharmacokinetic profile of the drug. With dose-dependent concentrations the half-life is about 12–13 hours, Tmax 1–2 hours and it is protein-bound, so the medication has a rapid absorption and minimal excretion by the kidney.[13]
Dapagliflozin disposition is not evidently affected by BMI or body weight, therefore the pharmacokinetic findings are expected to be applicable to patients with a higher BMI. Dapagliflozin resulted in dose-dependent increases excretions in urinary glucose, up to 47g/d following single-dose administration, which can be expected from its mechanism of action, dapagliflozin.[14]
In some long term clinical studies that have been made on dapagliflozin, dapagliflozin was associated with a decrease in body weight which was statistically superior compared to placebo or other active comparators. It is primarily associated with caloric rather than fluid loss.[14][31]
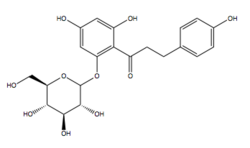
Structure-activity relationship
The Structure-activity relationship (SAR) of gliflozins is not fully understood.
The most common gliflozins are dapagliflozin, empagliflozin and canagliflozin. The differences in the structures is relatively small. The general structure includes a glucose sugar with an aromatic group in the β-position at the anomeric carbon. In addition to the glucose sugar moiety and the β-isomeric aryl substituent the aryl group is composed of a diarylmethylene structure.
The synthesis of Gliflozins involves three general steps. The first one is the construction of the aryl substituent, the next one is the introduction of the aryl moiety onto the sugar or glucosylation of the aryl substituent and the last one the deprotection and modification of the arylated anomeric center of the sugar.[36]
Phlorizin was the first type of gliflozin and it was non-selective against SGLT2/SGLT1. It is a natural O-aryl glycoside composed of a d-glucose and an aromatic ketone. However Phlorizin was very unstable, it is rapidly degraded by glucosidases in the small intestines, so it could not be used as a oral administration drug to treat diabetes. Structural modifications have been made to overcome this instability problem. The most efficient way was to conjugate aryl moiety with glucose moiety since C-glucosides are more stable in the small intestines than O-glucoside derivatives (C-C bond instead of C-O-C bond).[37]
In the sugar analogues of dapagliflozin, the β-C series are more active than α-C series so it is critical that the β-configuration is at C-1 for the inhibitory activity.[38] Both dapagliflozin and empagliflozin contain a chloride (Cl) group in its chemical structure. Cl is a halogen and it has a strong electron affinity. This electron affinity stabilizes the bonds and therefore it reduces the metabolism. The Cl group also reduces the IC50 value of the drug so the drug has better activity. The carbon-fluor bond (C-F) has also very strong electron affinity.[39]
For example in the chemical structure of canagliflozin a fluor is connected to an aromatic ring then the compound is more stable and the metabolism of the compound reduces. Empagliflozin contains tetrahydrofuran ring but not canagliflozin nor dapagliflozin.[40]
In the development of gliflozins the distal ring contains a thiazole ring instead of an aromatic ring. However the final chemical structures of the marketing gliflozins does not contain this thiazole ring.[41]
Alternative pharmacological actions
Gliflozins have been found to exhibit cardioprotective, renoprotective, anti‐hyperlipidemic, anti‐atherosclerotic, anti‐obesity, anti‐neoplastic, hepatoprotective, and renoprotective effects in in vitro, pre‐clinical, and clinical studies. These pleiotropic effects of this class have been attributed to a variety of its pharmacodynamic actions such as natriuresis, hemoconcentration, deactivation of renin-angiotensin-aldosterone system, ketone body formation, alterations in energy homeostasis, glycosuria, lipolysis, anti‐inflammatory, and anti‐oxidative actions.[42]
History
Phlorizin is a molecule with SGLT inhibiting properties, and served an important role in the development of the gliflozin class of drugs.
References
- ↑ 1.0 1.1 1.2 Shubrook, Jay; Baradar-Bokaie, Babak; Adkins, Sarah (2015). "Empagliflozin in the treatment of type 2 diabetes: Evidence to date". Drug Design, Development and Therapy 9: 5793–803. doi:10.2147/DDDT.S69926. PMID 26586935.
- ↑ Usman, Muhammad Shariq; Siddiqi, Tariq Jamal; Memon, Muhammad Mustafa; Khan, Muhammad Shahzeb; Rawasia, Wasiq Faraz; Talha Ayub, Muhammad; Sreenivasan, Jayakumar; Golzar, Yasmeen (2018). "Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: A systematic review and meta-analysis". European Journal of Preventive Cardiology 25 (5): 495–502. doi:10.1177/2047487318755531. PMID 29372664.
- ↑ Scheen, André J. (2014). "Pharmacodynamics, Efficacy and Safety of Sodium–Glucose Co-Transporter Type 2 (SGLT2) Inhibitors for the Treatment of Type 2 Diabetes Mellitus". Drugs 75 (1): 33–59. doi:10.1007/s40265-014-0337-y. PMID 25488697.
- ↑ Haas, B.; Eckstein, N.; Pfeifer, V.; Mayer, P.; Hass, M D S. (2014). "Efficacy, safety and regulatory status of SGLT2 inhibitors: Focus on canagliflozin". Nutrition & Diabetes 4 (11): e143. doi:10.1038/nutd.2014.40. PMID 25365416.
- ↑ 5.0 5.1 Clar, Christine; Gill, James Alexander; Court, Rachel; Waugh, Norman (2012). "Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes". BMJ Open 2 (5): e001007. doi:10.1136/bmjopen-2012-001007. PMID 23087012.
- ↑ Nauck, Michael (2014). "Update on developments with SGLT2 inhibitors in the management of type 2 diabetes". Drug Design, Development and Therapy 8: 1335–80. doi:10.2147/DDDT.S50773. PMID 25246775.
- ↑ Zheng, Sean L.; Roddick, Alistair J.; Aghar-Jaffar, Rochan; Shun-Shin, Matthew J.; Francis, Darrel; Oliver, Nick; Meeran, Karim (2018). "Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors with All-Cause Mortality in Patients with Type 2 Diabetes". JAMA 319 (15): 1580–1591. doi:10.1001/jama.2018.3024. PMID 29677303.
- ↑ 8.0 8.1 Hsia, Daniel S.; Grove, Owen; Cefalu, William T. (2016). "An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus". Current Opinion in Endocrinology, Diabetes and Obesity 24 (1): 73–79. doi:10.1097/MED.0000000000000311. PMID 27898586.
- ↑ Isaacs, Michelle; Tonks, Katherine T.; Greenfield, Jerry R. (2017). "Euglycaemic diabetic ketoacidosis in patients using sodium-glucose co-transporter 2 inhibitors". Internal Medicine Journal 47 (6): 701–704. doi:10.1111/imj.13442. PMID 28580740.
- ↑ Khouri, Charles; Cracowski, Jean-Luc; Roustit, Matthieu (2018). "SGLT-2 inhibitors and the risk of lower-limb amputation: Is this a class effect?". Diabetes, Obesity and Metabolism 20 (6): 1531–1534. doi:10.1111/dom.13255. PMID 29430814.
- ↑ BNF 73. Tavistock Square, London: BMJ Group. March–September 2017.
- ↑ Scheen, André J. (2014). "Drug–Drug Interactions with Sodium-Glucose Cotransporters Type 2 (SGLT2) Inhibitors, New Oral Glucose-Lowering Agents for the Management of Type 2 Diabetes Mellitus". Clinical Pharmacokinetics 53 (4): 295–304. doi:10.1007/s40262-013-0128-8. PMID 24420910. http://orbi.ulg.ac.be/handle/2268/164207.
- ↑ 13.0 13.1 13.2 Bhartia, Mithun; Tahrani, Abd A.; Barnett, Anthony H. (2011). "SGLT-2 Inhibitors in Development for Type 2 Diabetes Treatment". The Review of Diabetic Studies 8 (3): 348–354. doi:10.1900/RDS.2011.8.348. PMID 22262072.
- ↑ 14.0 14.1 14.2 Yang, Li; Li, Haiyan; Li, Hongmei; Bui, Anh; Chang, Ming; Liu, Xiaoni; Kasichayanula, Sreeneeranj; Griffen, Steven C. et al. (2013). "Pharmacokinetic and Pharmacodynamic Properties of Single- and Multiple-Dose of Dapagliflozin, a Selective Inhibitor of SGLT2, in Healthy Chinese Subjects". Clinical Therapeutics 35 (8): 1211–1222.e2. doi:10.1016/J.Clinthera.2013.06.017. PMID 23910664.
- ↑ "Drugs@FDA: FDA Approved Drug Products". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=204042. Retrieved 1 October 2018.
- ↑ "Invokana | European Medicines Agency" (in en). https://www.ema.europa.eu/medicines/human/EPAR/invokana. Retrieved 1 October 2018.
- ↑ "Drugs@FDA: FDA Approved Drug Products". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=202293. Retrieved 1 October 2018.
- ↑ "FDA approves Jardiance (empagliflozin) tablets for adults with type 2 diabetes". Boehringer Ingelheim / Eli Lilly and Company. 1 August 2014. Archived from the original on 5 November 2014. https://web.archive.org/web/20141105201008/http://us.boehringer-ingelheim.com/news_events/press_releases/press_release_archive/2014/08-01-14-fda-approves-jardiance-empagliflozin-tablets-reduce-blood-sugar-levels-adults-type-2-diabetes.html. Retrieved 5 November 2014.
- ↑ "FDA Advisory Committee recommends approval of Jardiance (empagliflozin) for cardiovascular indication in 12-11 vote". 28 June 2016. https://finance.yahoo.com/news/fda-advisory-committee-recommends-approval-222200556.html;_ylt=A0LEVrhllatXlP8AlncnnIlQ;_ylu=X3oDMTByOHZyb21tBGNvbG8DYmYxBHBvcwMxBHZ0aWQDBHNlYwNzcg--. Retrieved 10 August 2016.
- ↑ "Drugs@FDA: FDA Approved Drug Products". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=209803.
- ↑ "Approval of Suglat® Tablets, a Selective SGLT2 Inhibitor for Treatment of Type 2 Diabetes, in Japan". January 17, 2014. http://www.astellas.com/en/corporate/news/detail/approval-of-suglat-tablets-a-s.html.
- ↑ Poole, Raewyn M.; Dungo, Rosselle T. (26 March 2014). "Ipragliflozin: First Global Approval". Drugs 74 (5): 611–617. doi:10.1007/s40265-014-0204-x. PMID 24668021.
- ↑ Markham, Anthony; Elkinson, Shelley (2014). "Luseogliflozin: First Global Approval". Drugs 74 (8): 945–950. doi:10.1007/s40265-014-0230-8. PMID 24848756.
- ↑ 24.0 24.1 Da Silva, Paula Nogueira; Da Conceição, Raissa Alves; Do Couto Maia, Rodolfo; De Castro Barbosa, Maria Leticia (2018). "Sodium–glucose cotransporter 2 (SGLT-2) inhibitors: A new antidiabetic drug class". Medchemcomm 9 (8): 1273–1281. doi:10.1039/c8md00183a. PMID 30151080.
- ↑ Clinical trial number NCT02531035 for "A Phase 3 Study to Evaluate the Safety of Sotagliflozin in Patients With Type 1 Diabetes Who Have Inadequate Glycemic Control With Insulin Therapy Alone (inTandem3)" at ClinicalTrials.gov
- ↑ Poole, Raewyn M.; Prossler, Jennifer E. (2014). "Tofogliflozin: First Global Approval". Drugs 74 (8): 939–944. doi:10.1007/s40265-014-0229-1. PMID 24848755.
- ↑ Chao, E. C. (2014). "SGLT-2 Inhibitors: A New Mechanism for Glycemic Control". Clinical Diabetes 32 (1): 4–11. doi:10.2337/diaclin.32.1.4. PMID 26246672.
- ↑ 28.0 28.1 28.2 Anderson, Sarah L.; Marrs, Joel C. (2012). "Dapagliflozin for the Treatment of Type 2 Diabetes". Annals of Pharmacotherapy 46 (4): 590–598. doi:10.1345/aph.1Q538. PMID 22433611.
- ↑ 29.0 29.1 29.2 Li, An-Rong; Zhang, Jian; Greenberg, Joanne; Lee, Taeweon; Liu, Jiwen (2011). "Discovery of non-glucoside SGLT2 inhibitors". Bioorganic & Medicinal Chemistry Letters 21 (8): 2472–2475. doi:10.1016/j.bmcl.2011.02.056. PMID 21398124.
- ↑ Hummel, Charles S.; Lu, Chuan; Liu, Jie; Ghezzi, Chiari; Hirayama, Bruce A.; Loo, Donald D. F.; Kepe, Vladimir; Barrio, Jorge R. et al. (2012). "Structural selectivity of human SGLT inhibitors". American Journal of Physiology. Cell Physiology 302 (2): C373–C382. doi:10.1152/ajpcell.00328.2011. PMID 21940664.
- ↑ 31.0 31.1 31.2 Plosker, Greg L. (2012). "Dapagliflozin". Drugs 72 (17): 2289–2312. doi:10.2165/11209910-000000000-00000. PMID 23170914.
- ↑ "Jardiance". Drugs.com. https://www.drugs.com/pro/jardiance.html. Retrieved 31 October 2014.
- ↑ "Farxiga". Drugs.com. https://www.drugs.com/pro/farxiga.html. Retrieved 31 October 2014.
- ↑ "Invokana". Drugs.com. https://www.drugs.com/pro/invokana.html. Retrieved 31 October 2014.
- ↑ Madaan, Tushar; Akhtar, Mohd.; Najmi, Abul Kalam (2016). "Sodium glucose Co Transporter 2 (SGLT2) inhibitors: Current status and future perspective". European Journal of Pharmaceutical Sciences 93: 244–252. doi:10.1016/j.ejps.2016.08.025. PMID 27531551.
- ↑ LARSON, GERALD L. (March–April 2015). "The synthesis of gliflozins". Monographic Special Issue. http://www.teknoscienze.com/getpdf.php?filename=Contents%2FRiviste%2FPDF%2FCO2_Oligos-Peptides_2015_RGB.pdf&beginpage=39&endpage=42&filetitle=The%20synthesis%20of%20gliflozins.
- ↑ Chen, Zeng-Hao; Wang, Ruo-Wen; Qing, Feng-Ling (2012). "Synthesis and biological evaluation of SGLT2 inhibitors: Gem-difluoromethylenated Dapagliflozin analogs". Tetrahedron Letters 53 (17): 2171–2176. doi:10.1016/j.tetlet.2012.02.062. https://www.sciencedirect.com/science/article/pii/S0040403912002997.
- ↑ Ng, Wai-Lung; Li, Ho-Chuen; Lau, Kit-Man; Chan, Anthony K. N.; Lau, Clara Bik-San; Shing, Tony K. M. (17 July 2017). "Concise and Stereodivergent Synthesis of Carbasugars Reveals Unexpected Structure-Activity Relationship (SAR) of SGLT2 Inhibition" (in En). Scientific Reports 7 (1): 5581. doi:10.1038/s41598-017-05895-9. ISSN 2045-2322. PMID 28717146. PMC 5514135. https://www.nature.com/articles/s41598-017-05895-9.
- ↑ Ng, Wai-Lung; Li, Ho-Chuen; Lau, Kit-Man; Chan, Anthony K. N.; Lau, Clara Bik-San; Shing, Tony K. M. (2017). "Concise and Stereodivergent Synthesis of Carbasugars Reveals Unexpected Structure-Activity Relationship (SAR) of SGLT2 Inhibition". Scientific Reports 7 (1): 5581. doi:10.1038/s41598-017-05895-9. PMID 28717146. PMC 5514135. https://www.nature.com/articles/s41598-017-05895-9.
- ↑ "7.5: Electron Affinities". 18 November 2014. https://chem.libretexts.org/Textbook_Maps/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/07._Periodic_Properties_of_the_Elements/7.5%3A_Electron_Affinities. Retrieved 30 September 2018.
- ↑ Song, Kwang-Seop; Lee, Suk Ho; Kim, Min Ju; Seo, Hee Jeong; Lee, Junwon; Lee, Sung-Han; Jung, Myung Eun; Son, Eun-Jung et al. (2010). "Synthesis and SAR of Thiazolylmethylphenyl Glucoside as Novel C-Aryl Glucoside SGLT2 Inhibitors". ACS Medicinal Chemistry Letters 2 (2): 182–187. doi:10.1021/ml100256c. PMID 24900297.
- ↑ Madaan, Tushar; Husain, Ibraheem; Akhtar, Mohamad; Najmi, Abul Kalam (2018). "Exploring novel pharmacotherapeutic applications and repurposing potential of sodium glucose Co Transporter 2 inhibitors". Clinical and Experimental Pharmacology and Physiology 45 (9): 897–907. doi:10.1111/1440-1681.12963. PMID 29751356.