Biology:Omarigliptin
From HandWiki
Short description: Chemical compound
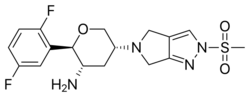 | |
| Clinical data | |
|---|---|
| Other names | MK-3102 |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H20F2N4O3S |
| Molar mass | 398.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Omarigliptin (MK-3102) is a potent, long-acting oral antidiabetic drug of the DPP-4 inhibitor class used for once-weekly treatment of type 2 diabetes and currently under development by Merck & Co.[1] It inhibits DPP-4 to increase incretin levels (GLP-1 and GIP),[2][3][4] which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying and decreases blood glucose levels.
History
Marizev (omarigliptin) 25 mg and 12.5 mg tablets were approved by Japan's Pharmaceuticals and Medical Devices Agency (PMDA) on 28th Sept 2015. Japan was the first country to have approved omarigliptin.[5] However Merck has announced that the company will not submit marketing application in the US and Europe.[6]
References
- ↑ "Pharmacokinetics of omarigliptin (MK‐3102), a once weekly dipeptidyl peptidase‐IV (DPP‐4) inhibitor, in patients with renal impairment.". Clin Pharmacol Ther (Merck & Co., Inc.) 95 (S1): S90. March 2014. https://celerion.com/wordpress/wp-content/uploads/2014/04/Celerion_ASCPT-2014_Pharmacokinetics-of-Omarigliptin-Mk-3102-in-Patients-With-Renal-Impairment.pdf. Retrieved 20 September 2015.
- ↑ "Dipeptidyl peptidase IV inhibitors: how do they work as new antidiabetic agents?". Regulatory Peptides 128 (2): 159–65. June 2005. doi:10.1016/j.regpep.2004.06.001. PMID 15780435.
- ↑ "Glucagon-like peptide 1 improved glycemic control in type 1 diabetes". BMC Endocrine Disorders 3 (1): 3. April 2003. doi:10.1186/1472-6823-3-3. PMID 12697069.
- ↑ "Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM". Diabetes 44 (6): 626–30. June 1995. doi:10.2337/diabetes.44.6.626. PMID 7789625.
- ↑ "Merck MARIZEV Once-Weekly DPP-4 Inhibitor For Type2 Diabetes Approved In Japan". NASDAQ. 28 September 2015. http://www.nasdaq.com/article/merck-marizevonceweekly-dpp4-inhibitor-for-type2-diabetes-approved-in-japan-20150928-00333.
- ↑ "Merck Provides Update on Filing Plans for Omarigliptin, an Investigational DPP-4 Inhibitor for Type 2 Diabetes". Merck Sharp & Dohme Corp.. April 8, 2016. https://www.mrknewsroom.com/news/company-statements/merck-provides-update-filing-plans-omarigliptin-investigational-dpp-4-inhibi.
See also
 |

