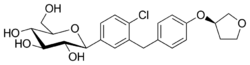
Chemistry:Empagliflozin
 | |
| Clinical data | |
|---|---|
| Trade names | Jardiance, others |
| Other names | BI-10773 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614043 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Sodium-glucose cotransporter-2 (SGLT2) inhibitor[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H27ClO7 |
| Molar mass | 450.91 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Empagliflozin, sold under the brand name Jardiance, among others, is an antidiabetic medication used to improve glucose control in people with type 2 diabetes.[8][2][10] It is not recommended for type 1 diabetes.[2][11] It is taken by mouth.[2]
Common side effects include hyperventilation, anorexia, abdominal pain, nausea, vomiting, lethargy, mental status changes, hypotension, acute kidney injury, and vaginal yeast infections.[2] Rarer but more serious side effects include a skin infection of the groin called Fournier's gangrene and a form of diabetic ketoacidosis with normal blood sugar levels.[2][12] Use during pregnancy or breastfeeding is not recommended.[13] Cautiously use empagliflozin with frequent monitoring of renal function in those with significant kidney disease, the use of empagliflozin for merely hyperglycaemia is not currently recommended given the risk of adverse events. The use of empagliflozin in patients with known cardiovascular disease may be beneficial, but risk and benefit ratio need to be considered and initiation of therapy should be individualised. It was also shown to help slow the progression of mild kidney problems.
Empagliflozin is an inhibitor of the sodium glucose co-transporter-2 (SGLT-2), and works by increasing sugar loss in urine.[2]
Empagliflozin was approved for medical use in the United States and in the European Union in 2014.[9][14][15] It is on the World Health Organization's List of Essential Medicines.[16] In 2021, it was the 85th most commonly prescribed medication in the United States, with more than 8 million prescriptions.[17] It has received approval as a generic medication from the US Food and Drug Administration (FDA).[18]
Medical uses
Empagliflozin is indicated in adults with heart failure (both reduced and preserved ejection fraction) to reduce the risk of hospitalisation for heart failure and to a lesser extent, cardiovascular death; in adults with type 2 diabetes and established cardiovascular disease to reduce the risk of cardiovascular death; and as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.[8][11][19]
In June 2023, the US Food and Drug Administration (FDA) expanded the indication, as an addition to diet and exercise, to improve blood sugar control in children 10 years and older with type 2 diabetes.[20]
Contraindications
- History of a severe allergic reaction to empagliflozin[8]
- End-stage kidney disease[8]
- Diabetic ketoacidosis[8]
Side effects
Common
- Empagliflozin increases the risk of genital fungal infections. The risk is highest in people with a prior history of genital fungal infections.[21]
- Empagliflozin has been thought to be associated with increased risk of urinary tract infections. Cumulative data from clinical trials have shown comparable risks for developing a UTI between placebo and empagliflozin. [citation needed]
- Empagliflozin reduces systolic and diastolic blood pressure and can increase the risk of low blood pressure, which can cause fainting and/or falls.[21] The risk is higher in older people, people taking diuretics, and people with reduced kidney function.[21]
- Slight increases in Low-density lipoprotein (LDL) cholesterol can be seen with empagliflozin, in the range of 2–4% from baseline.[21]
Serious
- Diabetic ketoacidosis, a rare but potentially life-threatening condition, may occur more commonly with empagliflozin and other SGLT-2 inhibitors.[22][23] While diabetic ketoacidosis is usually associated with elevated blood glucose levels, in people taking SGLT-2 inhibitors diabetic ketoacidosis may be seen with uncharacteristically normal blood glucose levels, a phenomenon called euglycemic diabetic ketoacidosis.[22] The absence of elevated blood glucose levels in people on an SGLT-2 inhibitor may make it more difficult to diagnose diabetic ketoacidosis. The risk of empagliflozin-associated euglycemic diabetic ketoacidosis may be higher in the setting of illness, dehydration, surgery, and/or alcohol consumption.[22] It is also seen in type 1 diabetes who take empagliflozin, which notably is an unapproved or "off-label" use of the medication.[23] To lessen the risk of developing ketoacidosis (a serious condition in which the body produces high levels of blood acids called ketones) after surgery, the FDA has approved changes to the prescribing information for SGLT2 inhibitor diabetes medicines to recommend they be stopped temporarily before scheduled surgery. Empagliflozin should each be stopped at least three days before scheduled surgery.[24] Symptoms of diabetic ketoacidosis include nausea, vomiting, abdominal pain, tiredness, and trouble breathing.[24]
- Fournier's gangrene, a rare but serious infection of the groin, occurs more commonly in people taking empagliflozin and other SGLT-2 inhibitors.[2][12] Symptoms include feverishness, a general sense of malaise, and pain or swelling around the genitals or in the skin behind them. The infection progresses quickly and urgent medical attention is recommended.[12]
- Empagliflozin can increase the risk of low blood sugar when it is used together with a sulfonylurea or insulin.[25] When used by itself or in addition to metformin it does not appear to increase the risk of hypoglycemia.[26]
Mechanism of action
Empagliflozin is an inhibitor of the sodium glucose co-transporter-2 (SGLT-2), which is found almost exclusively in the proximal tubules of nephronic components in the kidneys. SGLT-2 accounts for about 90 percent of glucose reabsorption into the blood. Blocking SGLT-2 reduces blood glucose by blocking glucose reabsorption in the kidney and thereby excreting glucose (i.e., blood sugar) via the urine.[27][28][29]
History
It was developed by Boehringer Ingelheim and is co-marketed by Eli Lilly and Company. It is also available as the combinations empagliflozin/linagliptin, empagliflozin/metformin, and empagliflozin/linagliptin/metformin.
For cardiovascular death, the FDA based its decision on a postmarketing study it required when it approved empagliflozin in 2014 as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.[14][11] Empagliflozin was studied in a postmarket clinical trial of more than 7,000 participants with type 2 diabetes and cardiovascular disease.[11] In the trial, empagliflozin was shown to reduce the risk of cardiovascular death compared to a placebo when added to standard of care therapies for diabetes and atherosclerotic cardiovascular disease.[11]
For heart failure, the safety and effectiveness of empagliflozin were evaluated by the FDA as an adjunct to standard of care therapy in a randomized, double-blind, international trial comparing 2,997 participants who received empagliflozin, 10 mg, once daily to 2,991 participants who received the placebo.[19] The main efficacy measurement was the time to death from cardiovascular causes or need to be hospitalized for heart failure.[19] Of the individuals who received empagliflozin for an average of about two years, 14% died from cardiovascular causes or were hospitalized for heart failure, compared to 17% of the participants who received the placebo.[19] This benefit was mostly attributable to fewer participants being hospitalized for heart failure.[19]
The FDA granted the application for empagliflozin priority review and granted the approval of Jardiance to Boehringer Ingelheim.[19]
Legal status
As of May 2013, Boehringer and Lilly had submitted applications for marketing approval to the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA).[30] The drug was approved in the European Union in May 2014,[9] and was approved in the United States in August 2014.[14][15][31] The FDA required four postmarketing studies: a cardiovascular outcomes trial, two studies in children, and a toxicity study in animals related to the pediatric trials.[14][31]
Research
A single study shows that half a tablet of empagliflozin 25 mg has similar glycemic efficacy as a full tablet.[32]
Weight and blood pressure
Empagliflozin causes moderate reductions in blood pressure and body weight. These effects are likely due to the excretion of glucose in the urine and a slight increase in urinary sodium excretion.[21][33] In clinical trials, patients taking empagliflozin lost an average of 2% of their baseline body weight.[34] A higher percentage of people taking empagliflozin achieved weight loss greater than 5% from their baseline.[21] The medication reduced systolic blood pressure by 3 to 5 millimeters of mercury (mmHg).[21] The effects on blood pressure and body weight are generally viewed as favorable, as many patients with type 2 diabetes have high blood pressure or are overweight or obese.[26][35]
References
- ↑ "Empagliflozin (Jardiance) Use During Pregnancy". 30 August 2018. https://www.drugs.com/pregnancy/empagliflozin.html.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "Empagliflozin Monograph for Professionals". AHFS. https://www.drugs.com/monograph/empagliflozin.html.
- ↑ "AusPAR: Empagliflozin". 8 November 2017. https://www.tga.gov.au/auspar/auspar-empagliflozin-0.
- ↑ JARDIANCE (Boehringer Ingelheim Pty Ltd)
- ↑ "Prescription medicines: registration of new chemical entities in Australia, 2014". 21 June 2022. https://www.tga.gov.au/resources/resource/guidance/prescription-medicines-registration-new-chemical-entities-australia-2014.
- ↑ "Jardiance 10 mg film-coated tablets – Summary of Product Characteristics (SmPC)". https://www.medicines.org.uk/emc/product/5441/smpc.
- ↑ "Jardiance 25 mg film-coated tablets – Summary of Product Characteristics (SmPC)". 23 October 2019. https://www.medicines.org.uk/emc/product/7703/smpc.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 "Jardiance- empagliflozin tablet, film coated". 22 January 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=faf3dd6a-9cd0-39c2-0d2e-232cb3f67565.
- ↑ 9.0 9.1 9.2 "Jardiance EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/jardiance.
- ↑ "Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)". Diabetologia 61 (12): 2461–2498. December 2018. doi:10.1007/s00125-018-4729-5. PMID 30288571.
- ↑ 11.0 11.1 11.2 11.3 11.4 "FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes" (Press release). U.S. Food and Drug Administration (FDA). 6 December 2016. Archived from the original on 11 February 2020. Retrieved 12 December 2016.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 12.0 12.1 12.2 "FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes". 9 February 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrences-serious-infection-genital-area-sglt2-inhibitors-diabetes.
- ↑ British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 691. ISBN 9780857113382.
- ↑ 14.0 14.1 14.2 14.3 "FDA approves Jardiance to treat type 2 diabetes" (Press release). U.S. Food and Drug Administration (FDA). 1 August 2014. Archived from the original on 22 October 2016. Retrieved 5 February 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 15.0 15.1 "Drug Approval Package: Jardiance (empagliflozin) Tablets NDA #204629". 8 September 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/204629Orig1s000TOC.cfm.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "2022 First Generic Drug Approvals". U.S. Food and Drug Administration (FDA). 3 March 2023. https://www.fda.gov/drugs/drug-and-biologic-approval-and-ind-activity-reports/2022-first-generic-drug-approvals.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 "FDA Approves Treatment for Wider Range of Patients with Heart Failure" (Press release). U.S. Food and Drug Administration (FDA). 24 February 2022. Archived from the original on 27 February 2022. Retrieved 27 February 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "FDA Approves New Class of Medicines to Treat Pediatric Type 2 Diabetes". U.S. Food and Drug Administration (FDA) (Press release). 20 June 2023. Archived from the original on 21 June 2023. Retrieved 20 June 2023.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 21.6 Khachikian, Deb (October 2015). "Empagliflozin (Jardiance) National Drug Monograph" (in en-US). U.S. Department of Veterans Affairs. https://www.pbm.va.gov/PBM/clinicalguidance/drugmonographs/Empagliflozin_Monograph.pdf.
- ↑ 22.0 22.1 22.2 "Euglycemic diabetic ketoacidosis: A predictable, detectable, and preventable safety concern with SGLT2 inhibitors". Diabetes Care 38 (9): 1638–1642. September 2015. doi:10.2337/dc15-1380. PMID 26294774.
- ↑ 23.0 23.1 "American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement on the Association of Sglt-2 Inhibitors and Diabetic Ketoacidosis". Endocrine Practice 22 (6): 753–762. June 2016. doi:10.4158/EP161292.PS. PMID 27082665.
- ↑ 24.0 24.1 "FDA revises labels of SGLT2 inhibitors for diabetes to include warning". U.S. Food and Drug Administration (FDA). 19 March 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Empagliflozin in combination therapy for treating type 2 diabetes". 25 March 2015. https://www.nice.org.uk/guidance/ta336/chapter/2-The-technology.
- ↑ 26.0 26.1 "Empagliflozin: Role in treatment options for patients with type 2 diabetes mellitus". Diabetes Therapy 8 (1): 33–53. February 2017. doi:10.1007/s13300-016-0211-x. PMID 27837465.
- ↑ "Inhibition of renal glucose reabsorption: A novel strategy for achieving glucose control in type 2 diabetes mellitus". Endocrine Practice 14 (6): 782–790. September 2008. doi:10.4158/ep.14.6.782. PMID 18996802.
- ↑ "Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus". The Journal of Clinical Endocrinology and Metabolism 95 (1): 34–42. January 2010. doi:10.1210/jc.2009-0473. PMID 19892839.
- ↑ "From victim to ally: The kidney as an emerging target for the treatment of diabetes mellitus". Current Medical Research and Opinion 25 (3): 671–681. March 2009. doi:10.1185/03007990802710422. PMID 19232040.
- ↑ Tucker, Miriam E. (7 May 2013). "First details of empagliflozin trials follow US and EU filings". http://www.medscape.com/viewarticle/803713.
- ↑ 31.0 31.1 Mechatie, Elizabeth (1 August 2014). "FDA approves empagliflozin for adults with type 2 diabetes". Clinical Endocrinology News Digital Network. http://www.clinicalendocrinologynews.com/home/article/fda-approves-empagliflozin-for-adults-with-type-2-diabetes/e8e500442424cf39148f889d6fe6b16c.html.
- ↑ "Treatment outcomes of graded dose of empagliflozin in type-2 diabetes: A real world study.". Ann Afr Med 21 (1): 26–33. March 2022. doi:10.4103/aam.aam_69_20. PMID 35313401.
- ↑ "SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review". Diabetologia 61 (10): 2108–2117. October 2018. doi:10.1007/s00125-018-4670-7. PMID 30132036.
- ↑ "Empagliflozin (Jardiance) for type 2 diabetes mellitus". American Family Physician 94 (12): 1014–1015. December 2016. PMID 28075091. https://www.aafp.org/afp/2016/1215/p1014.html.
- ↑ "Obesity and overweight fact sheet". World Health Organization. https://www.who.int/dietphysicalactivity/media/en/gsfs_obesity.pdf.
 |

