Chemistry:Tesaglitazar
From HandWiki
Short description: Chemical compound
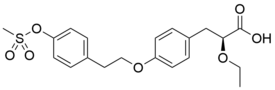 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H24O6S |
| Molar mass | 392.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tesaglitazar (also known as AZ 242) is a dual peroxisome proliferator-activated receptor agonist with affinity to PPARα and PPARγ, proposed for the management of type 2 diabetes.[1]
The drug had completed several phase III clinical trials,[2] however in May, 2006 AstraZeneca announced that it had discontinued further development.[3]
Cardiac toxicity of tesaglitazar is related to mitochondrial toxicity caused by decrease in PPARγ coactivator 1-α (PPARGC1A, PGC1α) and sirtuin 1 (SIRT1).[4]
References
- ↑ "Tesaglitazar, as add-on therapy to sulphonylurea, dose-dependently improves glucose and lipid abnormalities in patients with type 2 diabetes". Diabetes & Vascular Disease Research 4 (3): 194–203. September 2007. doi:10.3132/dvdr.2007.040. PMID 17907109.
- ↑ "GALIDA (tesaglitazar) Clinical Trial Report Summaries". AstraZeneca. http://www.astrazenecaclinicaltrials.com/ncmprintchapter.aspx?type=article¶m=520974. [|permanent dead link|dead link}}]
- ↑ "AstraZeneca Discontinues Development of GALIDA (tesaglitazar)". AstraZeneca. 2006-05-04. http://www.astrazeneca.com/Media/Press-releases/Article/20060504--AstraZeneca-Discontinues-Development-of-GALIDA-TM-te.
- ↑ "Dual peroxisome-proliferator-activated-receptor-α/γ activation inhibits SIRT1-PGC1α axis and causes cardiac dysfunction". JCI Insight 5 (17). August 2019. doi:10.1172/jci.insight.129556. PMID 31393858.
 |

