Biology:Evogliptin
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Suganon |
| Other names | DA-1229 |
| Routes of administration | By mouth |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
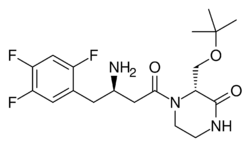
| Formula | C19H26F3N3O3 |
| Molar mass | 401.430 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Evogliptin (INN; trade names Suganon, Evodine) is an antidiabetic drug in the dipeptidyl peptidase-4 (DPP-4) inhibitor or "gliptin" class of drugs.[1] It was developed by the South Korea n pharmaceutical company Dong-A ST and is approved for use in South Korea[2] and Russia.[3] In a meta-analysis involving data from 6 randomized controlled trials (887 patients), Dutta et. al. demonstrated the good glycaemic efficacy and safety of this medicine as compared to other DPP4 inhibitors like sitagliptin and linagliptin. [4]
References
- ↑ "Evogliptin: First Global Approval". Drugs 75 (17): 2045–9. November 2015. doi:10.1007/s40265-015-0496-5. PMID 26541763.
- ↑ "Dong-A ST's DPP4 inhibitor, SUGANON, got approved for type 2 diabetes in Korea". pipelinereview.com. October 2, 2015. https://www.pipelinereview.com/index.php/2015100259148/Small-Molecules/Dong-A-STs-DPP4-inhibitor-SUGANON-got-approved-for-type-2-diabetes-in-Korea.html.
- ↑ "Evodine (evogliptin) film-coated tablets. Full prescribing information" (in Russian). http://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=6c9be7e5-6693-4b07-9f31-0175437b053e&t=.
- ↑ "Efficacy and Safety of Novel Dipeptidyl-Peptidase-4 Inhibitor Evogliptin in the Management of Type 2 Diabetes Mellitus: A Meta-Analysis.". Indian J Endocrinol Metab 24 (5): 434–445. Nov 2020. doi:10.4103/ijem.IJEM_418_20. PMID 33489850.
 |
