Chemistry:Muraglitazar
 | |
| Clinical data | |
|---|---|
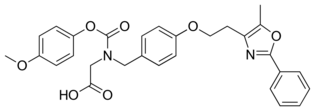
| Other names | 2-[(4-Methoxyphenoxy)carbonyl-[[4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]phenyl]methyl]amino]acetic acid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C29H28N2O7 |
| Molar mass | 516.550 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Muraglitazar (proposed tradename Pargluva) is a dual peroxisome proliferator-activated receptor agonist with affinity to PPARα and PPARγ.[1]
The drug had completed phase III clinical trials,[2] however in May 2006 Bristol-Myers Squibb announced that it had discontinued further development.[3]
Data on muraglitazar is relatively sparse due to the brief introduction and subsequent abandonment of this agent. One double-blind randomized clinical trial[2] comparing muraglitazar and pioglitazone found that the effects of the former were favourable in terms of HDL-C increase, decrease in total cholesterol, apolipoprotein B, triglycerides and a greater reduction in HbA1c (p <0.0001 for all comparisons). However, the muraglitazar group had a higher all-cause mortality, greater incidence of edema and heart failure and more weight gain compared to the pioglitazone group. A meta-analysis of the phase II and III clinical trials of muraglitazar revealed that it was associated with a greater incidence of myocardial infarction, stroke, transient ischemic attacks and congestive heart failure (CHF) when compared to placebo or pioglitazone.[4]
By calling attention to adverse events made public through the FDA advisory committee process, Dr Nissen came upon a mechanism to steer FDA from the outside.[citation needed] This mechanism came to fruition with rosiglitazone (Avandia) and led to FDA requiring demonstration of cardiac safety for new drugs to treat type 2 diabetes.[citation needed] This process is described by Dr Robert Misbin in INSULIN-History from an FDA Insider, published June 1, 2020 on Amazon.[promotional language]
References
- ↑ "Nonclinical safety evaluation of muraglitazar, a novel PPARalpha/gamma agonist". Toxicological Sciences 100 (1): 248–58. November 2007. doi:10.1093/toxsci/kfm193. PMID 17675651.
- ↑ 2.0 2.1 "Improvement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (alpha/gamma) peroxisome proliferator-activated receptor activator, in patients with type 2 diabetes inadequately controlled with metformin monotherapy: A double-blind, randomized, pioglitazone-comparative study". Diabetes Care 29 (5): 1016–23. May 2006. doi:10.2337/diacare.2951016. PMID 16644631. http://care.diabetesjournals.org/content/diacare/29/5/1016.full.pdf.
- ↑ "Bristol-Myers Squibb Announces Discontinuation of Development of Muraglitazar, an Investigational Oral Treatment for Type 2 Diabetes". PR Newswire from Bristol-Myers Squibb. May 18, 2006. http://www.prnewswire.com/news-releases/bristol-myers-squibb-announces-discontinuation-of-development-of-muraglitazar-an-investigational-oral-treatment-for-type-2-diabetes-56462702.html.
- ↑ "Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus". JAMA 294 (20): 2581–6. November 2005. doi:10.1001/jama.294.20.joc50147. PMID 16239637.
 |

