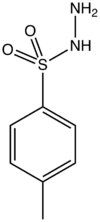
Chemistry:P-Toluenesulfonyl hydrazide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
4-methylbenzenesulfonohydrazide
| |
| Other names
tosyl hydrazide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Appearance | white solid |
| Melting point | 108–110 °C (226–230 °F; 381–383 K) |
| Hazards | |
| GHS pictograms |     
|
| GHS Signal word | Danger |
| H242, H301, H302, H315, H317, H319, H341, H373, H410 | |
| P201, P202, P210, P220, P234, P260, P261, P264, P270, P272, P273, P280, P281, P301+310, P301+312, P302+352, P305+351+338, P308+313, P314, P321, P330, P332+313, P333+313, P337+313, P362 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
p-Toluenesulfonyl hydrazide is the organic compound with the formula CH3C6H4SO2NHNH2. It is a white solid that is soluble in many organic solvents but not water or alkanes. It is a reagent in organic synthesis.[1]
Reactions
With ketones and aldehydes, it condenses to give the hydrazones:
- CH3C6H4SO2NHNH2 + R2C=O → CH3C6H4SO2NHN=CR2 + H2O
Upon heating in solution, it degrades, releasing diimide (N2H2), a useful reducing agent. Triisopropylbenzenesulfonylhydrazide is far more useful for this reaction.
Synthesis
Toluenesulfonyl hydrazide is prepared by the reaction of a toluenesulfonyl chloride with hydrazine:[2]
- CH3C6H4SO2Cl + 2 NH2NH2 → CH3C6H4SO2NHNH2 + + [NH2NH3]Cl
Reactions
Tosylhydrazides can be installed by nucleophilic attack and later removed by base. It thus provides a way to covert C-Cl to C-H.[3]
References
- ↑ Chamberlin, A. Richard; Sheppeck, James E.; Goess, Brian; Lee, Chulbom (2007). "P-Toluenesulfonylhydrazide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt137.pub2. ISBN 978-0471936237.
- ↑ Friedman, Lester; Litle, Robert L.; Reichle, Walter R. (1960). "p-Toluenesulfonylhydrazide". Org. Synth. 40: 93. doi:10.15227/orgsyn.040.0093.
- ↑ W. L. F. Armarego (1967). "Halogenoquinazolines". in W. L. F. Armarego. Chemistry of Heterocyclic Compounds. pp. 11–38. doi:10.1002/9780470186916.ch7. ISBN 9780470186916.
 |


