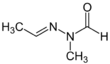
Chemistry:Gyromitrin
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
N′-Ethylidene-N-methylformohydrazide
| |||
| Other names
Acetaldehyde methylformylhydrazone
Formic acid 2-ethylidene-1-methylhydrazide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1922396 | |||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
| MeSH | Gyromitrin | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C 4H 8N 2O | |||
| Molar mass | 100.121 g·mol−1 | ||
| Boiling point | 143 °C (289 °F; 416 K) | ||
| Hazards | |||
| Main hazards | Toxic | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Gyromitrin is a toxin and carcinogen present in several members of the fungal genus Gyromitra, like G. esculenta. Its formula is C
4H
8N
2O. It is unstable and is easily hydrolyzed to the toxic compound monomethylhydrazine CH
3NHNH
2. Monomethylhydrazine acts on the central nervous system and interferes with the normal use and function of vitamin B6. Poisoning results in nausea, stomach cramps, and diarrhea, while severe poisoning can result in convulsions, jaundice, or even coma or death. Exposure to monomethylhydrazine has been shown to be carcinogenic in small mammals.
History
Poisonings related to consumption of the false morel Gyromitra esculenta, a highly regarded fungus eaten mainly in Finland and by some in parts of Europe and North America, had been reported for at least a hundred years. Experts speculated the reaction was more of an allergic one specific to the consumer, or a misidentification, rather than innate toxicity of the fungus, due to the wide range in effects seen. Some would suffer severely or perish while others exhibited no symptoms after eating similar amounts of mushrooms from the same dish. Yet others would be poisoned after previously eating the fungus for many years without ill-effects.[1] In 1885, Böhm and Külz described helvellic acid, an oily substance they believed to be responsible for the toxicity of the fungus.[2] The identity of the toxic constituents of Gyromitra species eluded researchers until 1968, when N-methyl-N-formylhydrazone was isolated by German scientists List and Luft and named gyromitrin. Each kilogram of fresh false morel had between 1.2 and 1.6 grams of the compound.[3][contradictory]
Mechanism of toxicity
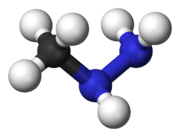
3NHNH
2), a toxic metabolite
Gyromitrin is a volatile, water-soluble hydrazine compound that can be hydrolyzed in the body into monomethylhydrazine (MMH) through the intermediate N-methyl-N-formylhydrazine.[4]

Other N-methyl-N-formylhydrazone derivatives have been isolated in subsequent research, although they are present in smaller amounts. These other compounds would also produce monomethylhydrazine when hydrolyzed, although it remains unclear how much each contributes to the false morel's toxicity.[5]
The toxins react with pyridoxal 5-phosphate—the activated form of pyridoxine—and form a hydrazone. This reduces production of the neurotransmitter GABA via decreased activity of glutamic acid decarboxylase,[6] which gives rise to the neurological symptoms. MMH also causes oxidative stress leading to methemoglobinemia.[7] Additionally during the metabolism of MMH, N-methyl-N-formylhydrazine is produced; this then undergoes cytochrome P450 regulated oxidative metabolism which via reactive nitrosamide intermediates leads to formation of methyl radicals which lead to liver necrosis.[8][9] Inhibition of diamine oxidase (histaminase) elevates histamine levels, resulting in headaches, nausea, vomiting, and abdominal pain.[10] Giving pyridoxine to rats poisoned with gyromitrin inhibited seizures, but did not prevent liver damage.
The toxicity of gyromitrin varies greatly according to the animal species being tested. Tests of administering gyromitrin to mice to observe the correlation between the formation of MMH and stomach pH have been performed. Higher levels of formed MMH were observed in the stomachs of the mice than were observed in control tests under less acidic conditions. The conclusions drawn were that the formation of MMH in a stomach is likely a result of acid hydrolysis of gyromitrin rather than enzymatic metabolism.[4] Based on this animal experimentation, it is reasonable to infer that a more acidic stomach environment would transform more gyromitrin into MMH, independent of the species in which the reaction is occurring.[4]
The median lethal dose (LD50) is 244 mg/kg in mice, 50–70 mg/kg in rabbits, and 30–50 mg/kg in humans.[11] The toxicity is largely due to the MMH that is created; about 35% of ingested gyromitrin is transformed to MMH.[12] Based on this conversion, the LD50 of MMH in humans has been estimated to be 1.6–4.8 mg/kg in children, and 4.8–8 mg/kg in adults.[11]
Occurrence and removal

Several Gyromitra species are traditionally considered very good edibles and several steps are available to remove gyromitrin from these mushrooms and allow their consumption. For North America, the toxin has been reliably reported from the species G. esculenta, G. gigas, and G. fastigiata. Species in which gyromitrin's presence is suspected, but not proven, include G. californica, G. caroliniana, G. korfii, and G. sphaerospora, in addition to Disciotis venosa and Sarcosphaera coronaria. The possible presence of the toxin renders these species "suspected, dangerous, or not recommended" for consumption.[13]
Gyromitrin content can differ greatly in different populations of the same species. For example, G. esculenta collected from Europe is "almost uniformly toxic", compared to rarer reports of toxicity from specimens collected from the US west of the Rocky Mountains.[14] A 1985 study reported that the stems of G. esculenta contained twice as much gyromitrin as the cap, and that mushrooms collected at higher altitudes contained less of the toxin than those collected at lower altitudes.[11]
The gyromitrin content in false morels has been reported to be in the range of 40–732 milligrams of gyromitrin per kilogram of mushrooms (wet weight).[15] Gyromitrin is volatile and water soluble, and can be mostly removed from the mushrooms by cutting them to small pieces and repeatedly boiling them in copious amounts of water under good ventilation. Prolonged periods of air drying also reduces levels of the toxin.[15] In the US, there are typically between 30 and 100 casesTemplate:How often of gyromitrin poisoning requiring medical attention. The mortality rate for cases worldwide is about 10%.[16]
Detection
The early methods developed for the determination of gyromitrin concentration in mushroom tissue were based on thin-layer chromatography and spectrofluorometry, or the electrochemical oxidation of hydrazine. These methods require large amounts of sample, are labor-intensive and unspecific. A 2006 study reported an analytical method based on gas chromatography-mass spectrometry with detection levels at the parts per billion level. The method, which involves acid hydrolysis of gyromitrin followed by derivatization with pentafluorobenzoyl chloride, has a minimum detectable concentration equivalent to 0.3 microgram of gyromitrin per gram of dry matter.[15]
Identification

When foraging for mushrooms in the wild, it is important to be cautious of ones that may not be safe to eat. Morel mushrooms are highly sought after; however, they can easily be confused with Gyromitra esculenta, also known as “false morels”. There are a few differing characteristics between the two species that can be used to avoid accidental poisoning. The cap of a real morel mushroom attaches directly to the stem, while the cap of a false morel grows around the stem. Real morel mushrooms are also hollow from top to bottom when cut in half, which varies from the filled nature of false morels. Finally, based on outward appearance, real morels are rather uniformly shaped and covered in pits that seem to fall inwards, whereas false morels are often considered more irregularly shaped with wavy ridges that seem to form outwards.[17]
Poisoning
Symptoms
The symptoms of poisoning are typically gastrointestinal and neurological.[18] Symptoms occur within 6–12 hours of consumption, although cases of more severe poisoning may present sooner—as little as 2 hours after ingestion. Initial symptoms are gastrointestinal, with sudden onset of nausea, vomiting, and watery diarrhea which may be bloodstained. Dehydration may develop if the vomiting or diarrhea is severe. Dizziness, lethargy, vertigo, tremor, ataxia, nystagmus, and headaches develop soon after;[18] fever often occurs, a distinctive feature which does not develop after poisoning by other types of mushrooms.[19] In most cases of poisoning, symptoms do not progress from these initial symptoms, and patients recover after 2–6 days of illness.[20]
In some cases there may be an asymptomatic phase following the initial symptoms which is then followed by more significant toxicity including kidney damage,[21] liver damage, and neurological dysfunction including seizures and coma.[7] These signs usually develop within 1–3 days in serious cases.[18] The patient develops jaundice and the liver and spleen become enlarged, in some cases blood sugar levels will rise (hyperglycemia) and then fall (hypoglycemia) and liver toxicity is seen. Additionally, intravascular hemolysis causes destruction of red blood cells resulting in increases in free hemoglobin and hemoglobinuria, which can lead to kidney toxicity or kidney failure. Methemoglobinemia may also occur in some cases. This is where higher than normal levels of methemoglobin—a form of hemoglobin that can not carry oxygen—are found in the blood. It causes the patient to become short of breath and cyanotic.[22] Cases of severe poisoning may progress to a terminal neurological phase, with delirium, muscle fasciculations and seizures, and mydriasis progressing to coma, circulatory collapse, and respiratory arrest.[23] Death may occur from five to seven days after consumption.[24]
Toxic effects from gyromitrin may also be accumulated from sub-acute and chronic exposure due to "professional handling"; symptoms include pharyngitis, bronchitis, and keratitis.[18]
Treatment
Treatment is mainly supportive; gastric decontamination with activated charcoal may be beneficial if medical attention is sought within a few hours of consumption. However, symptoms often take longer than this to develop, and patients do not usually present for treatment until many hours after ingestion, thus limiting its effectiveness.[25] Patients with severe vomiting or diarrhea can be rehydrated with intravenous fluids.[20] Monitoring of biochemical parameters such as methemoglobin levels, electrolytes, liver and kidney function, urinalysis, and complete blood count is undertaken and any abnormalities are corrected. Dialysis can be used if kidney function is impaired or the kidneys are failing. Hemolysis may require a blood transfusion to replace the lost red blood cells, while methemoglobinemia is treated with intravenous methylene blue.[26]
Pyridoxine, also known as vitamin B6, can be used to counteract the inhibition by MMH on the pyridoxine-dependent step in the synthesis of the neurotransmitter GABA. Thus GABA synthesis can continue and symptoms are relieved.[27] Pyridoxine, which is only useful for the neurological symptoms and does not decrease hepatic toxicity,[9][28] is given at a dose of 25 mg/kg; this can be repeated up to a maximum total of 15 to 30 g daily if symptoms do not improve.[29] Benzodiazepines are given to control seizures; as they also modulate GABA receptors they may potentially increase the effect of pyridoxine. Additionally MMH inhibits the chemical transformation of folic acid into its active form, folinic acid, this can be treated by folinic acid given at 20–200 mg daily.[7]
Toxicity controversy
Due to variances seen in the effects of consumption of the Gyromitra esculenta, there is some controversy surrounding its toxicity. Historically, there was some confusion over what was causing the symptoms to form after consuming the mushrooms. Over time, there were poisonings across Europe due to the consumption of Gyromitra mushrooms; however, the toxin causing the poisonings was unknown at that time. In 1793, mushroom poisonings that occurred in France were attributed to Morchella pleopus, and in 1885, the poisonings were said to be caused by “helvellic acid”. The identity of the toxin found in Gyromitra was not known until List and Luft of Germany were able to isolate and identify the structure of gyromitrin from these mushrooms in 1968.[30]
Gyromitrin may not be considered especially toxic, which may lead people to underestimate its poisonous qualities. In Poland, from 1953 to 1962, there were 138 documented poisonings, only two of which were fatal. Of 706 calls to the Swedish poison center regarding Gyromitra mushrooms between 1994 and 2002, there were no fatalities. In the United States from 2001 to 2011, 448 calls to poison centers involved gyromitrin. The North American Mycological Association (NAMA) reported on 27 cases over 30 years, none of which were fatal.[30] Although poisonings due to gyromitrin are not often fatal, it is still highly toxic to the liver.[31] Of those 27 analyzed cases, nine developed liver injury; there were also three instances of acute kidney injury.[30] As gyromitrin is not especially stable, most poisonings apparently occur from the consumption of the raw or insufficiently cooked "false morel" mushrooms.[31]
There are also possibly several strains of Gyromitra esculenta that vary from region to region and have differing levels of the toxin. For example, there is a less toxic variety that grows west of the Rockies in North America. The toxin may also diminish as the seasons change, as most exposures occur in the Spring.[30] This may help explain some conflicting reports on whether the fungus is edible or not.[31]
Carcinogenicity
Monomethylhydrazine,[32] as well as its precursors methylformylhydrazine[33][34] and gyromitrin[35] and raw Gyromitra esculenta,[36] have been shown to be carcinogenic in experimental animals.[37][38] Although Gyromitra esculenta has not been observed to cause cancer in humans,[39] it is possible there is a carcinogenic risk for people who ingest these types of mushrooms.[33] Even small amounts may have a carcinogenic effect.[40] At least 11 different hydrazines have been isolated from Gyromitra esculenta, and it is not known if the potential carcinogens can be completely removed by parboiling.[41]
References
- ↑ Benjamin, p. 264.
- ↑ "Über die giftigen Bestandstelle der giftigen Lorchel" (in German). Archives of Experimental Pathology and Pharmacology 19: 403. 1885.
- ↑ (in German) "[Gyromitrin, the poison of Gyromitra esculenta. 16. On the fungi contents]" (in German). Archiv der Pharmazie 301 (4): 294–305. 1968. doi:10.1002/ardp.19683010410. PMID 5244383.
- ↑ 4.0 4.1 4.2 Nagel, Donald; Wallcave, L.; Toth, Bela; Kupper, Robert (1977). "Formation of Methylhydrazine from Acetaldehyde N-Methyl-N-formylhydrazone, a Component of Gyromitra esculenta". Cancer Research 37 (9): 3458–60. PMID 18281.
- ↑ Pyysalo H. (1975). "Some new toxic compounds in false morels, Gyromitra esculenta". Naturwissenschaften 62 (8): 395. doi:10.1007/BF00625355. PMID 1238907. Bibcode: 1975NW.....62..395P.
- ↑ Cornish HH. (1969). "The role of vitamin B6 in the toxicity of hydrazines". Annals of the New York Academy of Sciences 166 (1): 136–45. doi:10.1111/j.1749-6632.1969.tb54264.x. PMID 5262010. Bibcode: 1969NYASA.166..136C.
- ↑ 7.0 7.1 7.2 "Poisoning by Gyromitra esculenta—a review". Journal of Applied Toxicology 11 (4): 235–43. 1991. doi:10.1002/jat.2550110403. PMID 1939997.
- ↑ "Indications for nitrosamide formation from the mushroom poison gyromitrin by rat liver microsomes". Xenobiotica 10 (7–8): 557–64. 1980. doi:10.3109/00498258009033790. PMID 7445522.
- ↑ 9.0 9.1 "Liver injury by the false morel poison gyromitrin". Toxicology 12 (2): 155–63. 1979. doi:10.1016/0300-483X(79)90042-8. PMID 473232.
- ↑ "N-methyl-N-formylhydrazine: a toxic and mutagenic inhibitor of the intestinal diamine oxidase". Agents and Actions 14 (3–4): 351–55. 1984. doi:10.1007/BF01973825. PMID 6428190.
- ↑ 11.0 11.1 11.2 "Variations of monomethylhydrazine content in Gyromitra esculenta". Mycologia 77 (2): 259–64. 1985. doi:10.2307/3793077.
- ↑ "Quantitative evaluation of the metabolic formation of methylhydrazine from acetaldehyde N-methyl-N-formylhydrazone, the main poisonous compound of Gyromitra esculenta". Toxicology Letters 2 (5): 261–65. 1978. doi:10.1016/0378-4274(78)90023-1.
- ↑ Poisonous Mushrooms of Canada: Including other Inedible Fungi. Markham, Ontario: Fitzhenry & Whiteside in cooperation with Agriculture Canada and the Canadian Government Publishing Centre, Supply and Services Canada. 1985. pp. 119–120. ISBN 978-0-88902-977-4.
- ↑ The New Savory Wild Mushroom. Seattle: University of Washington Press. 1987. pp. 219–20. ISBN 978-0-295-96480-5.
- ↑ 15.0 15.1 15.2 "Gas chromatography-mass spectrometry determination of the pentafluorobenzoyl derivative of methylhydrazine in false morel (Gyromitra esculenta) as a monitor for the content of the toxin gyromitrin". Journal of Chromatography A 1125 (2): 229–33. 2006. doi:10.1016/j.chroma.2006.05.040. PMID 16782115.
- ↑ Kuo M. (2005). Morels. Ann Arbor, MI: University of Michigan Press. pp. 23–27. ISBN 978-0-472-03036-1.
- ↑ "Morel Mushroom Identification". 24 February 2022. https://www.mushroom-appreciation.com/morel-mushroom.html#sthash.7vjFs3Lb.IVuD9MPl.dpbs.
- ↑ 18.0 18.1 18.2 18.3 "Cytotoxic fungi—an overview". Toxicon 42 (4): 339–49. 2003. doi:10.1016/S0041-0101(03)00238-1. PMID 14505933.
- ↑ Benjamin, p. 273.
- ↑ 20.0 20.1 Lampe KF. (1979). "Toxic fungi". Annual Review of Pharmacology and Toxicology 19: 85–104. doi:10.1146/annurev.pa.19.040179.000505. PMID 378111.
- ↑ "Renal functional response to the mushroom poison gyromitrin". Toxicology 13 (2): 187–96. 1979. doi:10.1016/s0300-483x(79)80022-0. PMID 42171.
- ↑ Benjamin, p. 274.
- ↑ "A case of fatal poisoning by Gyromitra esculenta". Archives of Toxicology 33 (1): 49–54. 1974. doi:10.1007/BF00297052. PMID 4480349.
- ↑ "Mushroom poisoning. Case reports and a review of therapy". JAMA 251 (8): 1057–61. 1984. doi:10.1001/jama.251.8.1057. PMID 6420582.
- ↑ Köppel C. (1993). "Clinical symptomatology and management of mushroom poisoning". Toxicon 31 (12): 1513–40. doi:10.1016/0041-0101(93)90337-I. PMID 8146866.
- ↑ Benjamin, p. 276.
- ↑ "Amelioration of toxic effects of ethylidene gyromitrin (false morel poison) with pyridoxine chloride". Journal of Food Safety 3 (3): 199–203. 1981. doi:10.1111/j.1745-4565.1981.tb00422.x.
- ↑ "Reversal of the toxicity of hydrazine an analogues by pyridoxine hydrochloride". Toxicology 7 (1): 31–36. 1977. doi:10.1016/0300-483X(77)90035-X. PMID 841582.
- ↑ "Treatment of hydrazine-induced coma with pyridoxine". New England Journal of Medicine 294 (17): 938–39. 1976. doi:10.1056/NEJM197604222941708. PMID 815813.
- ↑ 30.0 30.1 30.2 30.3 Horowitz, Keahi M.; Horowitz, B. Z. (2020). "Gyromitra Mushroom Toxicity". Gyromitra Mushroom Toxicity (Updated 2019). StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470580/.
- ↑ 31.0 31.1 31.2 Subramanian, C. V. (1995). "Mushrooms: Beauty, diversity, relevance". Current Science Association 69 (12): 986–998.
- ↑ "Methylhydrazine tumorigenesis in Syrian golden hamsters and the morphology of malignant histiocytomas". Cancer Research 33 (11): 2744–53. 1973. PMID 4355982.
- ↑ 33.0 33.1 "Tumors induced in mice by N-methyl-N-formylhydrazine of the false morel Gyromitra esculenta". Journal of the National Cancer Institute 60 (1): 201–04. 1978. doi:10.1093/jnci/60.1.201. PMID 628017.
- ↑ "False morel mushroom Gyromitra esculenta toxin: N-methyl-N-formylhdrazine carcinogenesis in mice". Mycopathologia 68 (2): 121–28. 1979. doi:10.1007/BF00441091. PMID 573857.
- ↑ "Cancer induction in mice with acetaldehyde methylformylhydrazone of the false morel mushroom". Journal of the National Cancer Institute 67 (4): 881–87. 1981. doi:10.1093/jnci/67.4.881. PMID 6944556.
- ↑ "Cancer induction in mice by feeding the raw false morel mushroom Gyromitra esculenta". Cancer Research 52 (8): 2279–84. 1992. PMID 1559231.
- ↑ "Methylation of deoxyribonucleic acid in the rat by the mushroom poison gyromitrin". Journal of Agricultural and Food Chemistry 31 (5): 1117–20. 1983. doi:10.1021/jf00119a048. PMID 6685148.
- ↑ "Methylation of rat and mouse DNA by the mushroom poison gyromitrin and its metabolite monomethylhydrazine". Cancer Letters 61 (2): 165–70. 1992. doi:10.1016/0304-3835(92)90175-U. PMID 1730140.
- ↑ A Colour Atlas of Poisonous Fungi. Wolfe Publishing. 1990. pp. 62–68. ISBN 978-0-7234-1576-3.
- ↑ Benjamin, pp. 128–29.
- ↑ Dart RC. (2004). "Mushrooms". Medical toxicology. Philadelphia, PA: Williams & Wilkins. pp. 1719–35. ISBN 978-0-7817-2845-4.
Books cited
- Benjamin, Denis R. (1995). Mushrooms: Poisons and Panaceas—a Handbook for Naturalists, Mycologists and Physicians. New York, NY: WH Freeman and Company. ISBN 978-0-7167-2600-5.
External links
- "Gyromitra esculenta, one of the false morels"
- Official Finnish instructions for the processing of false morels
- Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver
 |