Chemistry:Scandium nitride
| |
| Names | |
|---|---|
| IUPAC name
Scandium nitride
| |
| Other names
Azanylidynescandium
Nitridoscandium | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| ScN | |
| Molar mass | 58.963 |
| Density | 4.4 g/cm3 |
| Melting point | 2,600 °C (4,710 °F; 2,870 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H228 | |
| Related compounds | |
Other anions
|
Scandium phosphide Scandium arsenide Scandium antimonide Scandium bismuthide |
Other cations
|
Yttrium nitride Lutetium nitride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
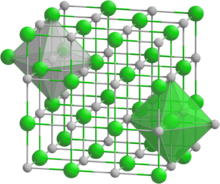
Scandium nitride (ScN) is a binary III-V indirect bandgap semiconductor. It is composed of the scandium cation and the nitride anion. It forms crystals that can be grown on tungsten foil through sublimation and recondensation.[1] It has a rock-salt crystal structure with octahedral bonding coordination. It exhibits lattice constant of 0.451 nm and an indirect bandgap of 0.9 eV and direct bandgap of 2 to 2.4 eV.[1][2] These crystals can be synthesized by dissolving nitrogen gas with indium-scandium melts, magnetron sputtering, Molecular Beam Epitaxy (MBE), HVPE and other deposition methods.[2][3] Scandium nitride is also an effective gate for semiconductors on a silicon dioxide (SiO2) or hafnium dioxide (HfO2) substrate.[4] Scandium nitride is the first nitride semiconductor reported to be synthesized without an active Nitrogen plasma source using the Molecular Beam Epitaxy (MBE) technique. It exhibits a scavenging effect, in which scandium at the growth front dissociates molecular nitrogen and incorporates it into the lattice.[5] Scandium nitride can be potentially used in thermoelectric materials as a semiconducting layer in epitaxial single-crystalline metal/semiconductor superlattices for thermoelectric, plasmonic and thermophotonic applications, and as a substrate material for high-quality GaN-based devices and other solid-state applications.[6]
References
- ↑ 1.0 1.1 Gu, Zheng; Edgar, J H; Pomeroy, J; Kuball, M; Coffey, D W (August 2004). "Crystal Growth and Properties of Scandium Nitride". Journal of Materials Science: Materials in Electronics 15 (8): 555–559. doi:10.1023/B:JMSE.0000032591.54107.2c.
- ↑ 2.0 2.1 Biswas, Bidesh; Saha, Bivas (2019-02-14). "Development of semiconducting ScN". Physical Review Materials 3 (2). doi:10.1103/physrevmaterials.3.020301. ISSN 2475-9953. Bibcode: 2019PhRvM...3b0301B.
- ↑ Zhang, Guodong; Kawamura, Fumio; Oshima, Yuichi; Villora, Encarnacion; Shimamura, Kiyoshi (4 August 2016). "Synthesis of Scandium Nitride Crystals from Indium–Scandium Melts". International Journal of Applied Ceramic Technology 13 (6): 1134–1138. doi:10.1111/ijac.12576.
- ↑ Yang, Hyundoek; Heo, Sungho; Lee, Dongkyu; Choi, Sangmoo; Hwang, Hyunsang (13 January 2006). "Effective Work Function of Scandium Nitride Gate Electrodes on SiO2 and HfO2". Japanese Journal of Applied Physics 45 (2): L83–L85. doi:10.1143/JJAP.45.L83. Bibcode: 2006JaJAP..45L..83Y.
- ↑ Savant, Chandrashekhar P.; Verma, Anita; Nguyen, Thai-Son; van Deurzen, Len; Chen, Yu-Hsin; He, Zhiren; Rezaie, Salva S.; Gollwitzer, Jakob et al. (2024-11-06). "Self-activated epitaxial growth of ScN films from molecular nitrogen at low temperatures". APL Materials 12 (11): 111108. doi:10.1063/5.0222995. ISSN 2166-532X.
- ↑ Shi, X.; Kong, H.; Li, C.-P.; Uher, C.; Yang, J.; Salvador, J. R.; Wang, H.; Chen, L. et al. (2008-05-05). "Low thermal conductivity and high thermoelectric figure of merit in n-type BaxYbyCo4Sb12 double-filled skutterudites" (in en). Applied Physics Letters 92 (18). doi:10.1063/1.2920210. ISSN 0003-6951. https://pubs.aip.org/apl/article/92/18/182101/334685/Low-thermal-conductivity-and-high-thermoelectric.
| NH3 | He(N2)11 | ||||||||||||||||
| Li3N | Be3N2 | BN | β-C3N4 g-C3N4 |
N2 | NxOy | NF3 | Ne | ||||||||||
| Na3N | Mg3N2 | AlN | Si3N4 | PN P3N5 |
SxNy SN S4N4 |
NCl3 | Ar | ||||||||||
| K3N | Ca3N2 | ScN | TiN | VN | CrN Cr2N |
MnxNy | FexNy | CoN | Ni3N | CuN | Zn3N2 | GaN | Ge3N4 | As | Se | NBr3 | Kr |
| Rb3N | Sr3N2 | YN | ZrN | NbN | β-Mo2N | Tc | Ru | Rh | PdN | Ag3N | CdN | InN | Sn | Sb | Te | NI3 | Xe |
| Cs3N | Ba3N2 | Hf3N4 | TaN | WN | Re | Os | Ir | Pt | Au | Hg3N2 | TlN | Pb | BiN | Po | At | Rn | |
| Fr3N | Ra3N | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | CeN | Pr | Nd | Pm | Sm | Eu | GdN | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UN | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |
