Chemistry:Germanium nitride
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Germanium(IV) nitride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| Ge3N4 | |
| Molar mass | 273.947 g/mol |
| Appearance | light brown powder |
| Density | 5.25 g/cm3 |
| Boiling point | 900 °C (1,650 °F; 1,170 K) (decomposes) |
| Related compounds | |
Other anions
|
Germanium phosphide |
Other cations
|
Silicon nitride Gallium nitride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Germanium(IV) nitride is an inorganic compound with the chemical formula Ge3N4. It can be produced through the reaction of germanium and ammonia:[1]
- 3 Ge + 4 NH3 → Ge3N4 + 6 H2
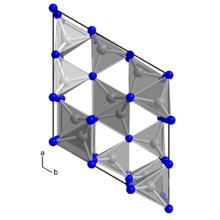
Structure
In its pure state, germanium(IV) nitride is a colorless, inert solid that crystallizes in many polymorphs, of which the most stable is the trigonal β-form (space group P31c). In this structure, the germanium atoms are tetrahedrally coordinated while the nitrogen atoms are trigonal planar.[2] The γ-form, which forms under high pressure, has a spinel structure.[3]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Ruddlesden, S. N.; Popper, P. (1958). "On the crystal structure of the nitrides of silicon and germanium". Acta Crystallographica 11 (7): 465–468. doi:10.1107/S0365110X58001304. http://scripts.iucr.org/cgi-bin/paper?S0365110X58001304.
- ↑ McMillan, Paul F.; Deb, Sudip K.; Dong, Jian-Jung (2003). "High-pressure metastable phase transitions in ?-Ge3N4 studied by Raman spectroscopy" (in en). Journal of Raman Spectroscopy 34 (7–8): 567–577. doi:10.1002/jrs.1007. ISSN 0377-0486. Bibcode: 2003JRSp...34..567M.
Template:Inorganic-compound-stub
Salts and covalent derivatives of the nitride ion
| NH3 | He(N2)11 | ||||||||||||||||
| Li3N | Be3N2 | BN | β-C3N4 g-C3N4 |
N2 | NxOy | NF3 | Ne | ||||||||||
| Na3N | Mg3N2 | AlN | Si3N4 | PN P3N5 |
SxNy SN S4N4 |
NCl3 | Ar | ||||||||||
| K3N | Ca3N2 | ScN | TiN | VN | CrN Cr2N |
MnxNy | FexNy | CoN | Ni3N | CuN | Zn3N2 | GaN | Ge3N4 | As | Se | NBr3 | Kr |
| Rb3N | Sr3N2 | YN | ZrN | NbN | β-Mo2N | Tc | Ru | Rh | PdN | Ag3N | CdN | InN | Sn | Sb | Te | NI3 | Xe |
| Cs3N | Ba3N2 | Hf3N4 | TaN | WN | Re | Os | Ir | Pt | Au | Hg3N2 | TlN | Pb | BiN | Po | At | Rn | |
| Fr3N | Ra3N | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | CeN | Pr | Nd | Pm | Sm | Eu | GdN | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UN | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |
