Chemistry:Zilucoplan
 | |
| Clinical data | |
|---|---|
| Trade names | Zilbrysq |
| Other names | RA101495 |
| License data |
|
| Routes of administration | Subcutaneous |
| Drug class | Complement inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
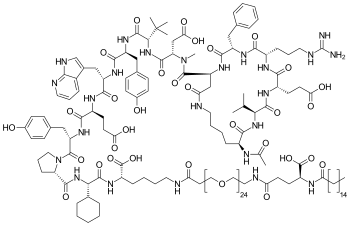
| Formula | C172H278N24O55 |
| Molar mass | 3562.229 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zilucoplan, sold under the brand name Zilbrysq, is a medication used for the treatment of generalized myasthenia gravis.[1][4][5] It is a complement inhibitor that is injected subcutaneously (under the skin).[1]
Zilucoplan is a cyclic peptide that binds to the protein complement component 5 (C5) and inhibits its cleavage into C5a and C5b.[6]
Zilucoplan was approved for medical use in the United States in October 2023,[1][7] and in the European Union in December 2023.[2]
Medical uses
Zilucoplan is indicated for the treatment of generalized myasthenia gravis in adults who are anti-acetylcholine receptor antibody positive.[1]
Society and culture
Legal status
In September 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Zilbrysq, intended for the treatment of myasthenia gravis.[8] The applicant for this medicinal product is UCB Pharma S.A.[8] Zilucoplan was approved for medical use in the Europea Union in December 2023.[2]
Zilucoplan was granted orphan drug designation by the US Food and Drug Administration (FDA) in August 2019,[9] and by the EMA in July 2022.[10]
Brand names
Zilucoplan is the international nonproprietary name.[11]
Zilucoplan is sold under the brand name Zilbrysq.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Zilbrysq (zilucoplan) injection, for subcutaneous use". https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216834s000lbl.pdf.
- ↑ 2.0 2.1 2.2 2.3 "Zilbrysq EPAR". 1 December 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/zilbrysq.
- ↑ "Zilbrysq Product information". 4 December 2023. https://ec.europa.eu/health/documents/community-register/html/h1764.htm.
- ↑ "RA101495, a subcutaneously administered peptide inhibitor of complement component 5 (C5) for the treatment of generalized myasthenia gravis (gMG): Phase 1 results and phase 2 design (S31. 006)". Neurology 90 (15 Supplement). April 2018. doi:10.1212/WNL.90.15_supplement.S31.006. https://n.neurology.org/content/90/15_Supplement/S31.006. Retrieved 24 June 2021.
- ↑ "Zilucoplan: An Investigational Complement C5 Inhibitor for the Treatment of Acetylcholine Receptor Autoantibody-Positive Generalized Myasthenia Gravis". Expert Opinion on Investigational Drugs 30 (5): 483–493. May 2021. doi:10.1080/13543784.2021.1897567. PMID 33792453.
- ↑ "Preclinical Evaluation of RA101495, a Potent Cyclic Peptide Inhibitor of C5 for the Treatment of Paroxysmal Nocturnal Hemoglobinuria". Blood 126 (23): 939. 2015. doi:10.1182/blood.V126.23.939.939.
- ↑ "Novel Drug Approvals for 2023". 22 December 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023.
- ↑ 8.0 8.1 "Zilbrysq: Pending EC decision". 15 September 2023. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/zilbrysq. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Zilucoplan Orphan Drug Designations and Approvals". https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=699319.
- ↑ "EU/3/22/2650: Orphan designation for the treatment of myasthenia gravis". 15 September 2023. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu-3-22-2650.
- ↑ "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 80". WHO Drug Information 32 (3). 2018.
External links
- Clinical trial number NCT04115293 for "Safety, Tolerability, and Efficacy of Zilucoplan in Subjects With Generalized Myasthenia Gravis (RAISE)" at ClinicalTrials.gov
 |

