Medicine:Diet and cancer
Dietary factors are recognized as having a significant effect on the risk of cancers, with different dietary elements both increasing and reducing risk. Diet and obesity may be related to up to 30–35% of cancer deaths,[1] while physical inactivity appears to be related to 7% risk of cancer occurrence.[2]
While many dietary recommendations have been proposed to reduce the risk of cancer, few have significant supporting scientific evidence.[3] Obesity and drinking alcohol have been correlated with the incidence and progression of some cancers.[3] Lowering the consumption of sweetened beverages is recommended as a measure to address obesity.[4]
Some specific foods are linked to specific cancers. There is strong evidence that processed meat and red meat intake increases risk of colorectal cancer.[5][6][7] Aflatoxin B1, a frequent food contaminant, increases risk of liver cancer,[8] while drinking coffee is associated with a reduced risk.[9] Betel nut chewing causes oral cancer.[8] Stomach cancer is more common in Japan due to its high-salt diet.[8][10] Immigrant communities tend to develop the risk of their new country, often within one generation, suggesting a substantial link between diet and cancer.[11]
Dietary recommendations for cancer prevention typically include weight management and eating a healthy diet, consisting mainly of "vegetables, fruit, whole grains and fish, and a reduced intake of red meat, animal fat, and refined sugar."[3] A healthy dietary pattern may lower cancer risk by 10-20%.[12]
Types of diet
Restrictive diets
A number of diets and diet-based regimes are claimed to be useful against cancer. Popular types of "anti-cancer" diets include the Breuss diet, Gerson therapy, the Budwig protocol and the macrobiotic diet. None of these diets has been found to be effective, and some of them have been found to be harmful.[13]
Dietary patterns
Nutritional epidemiologists use multivariate statistics, such as principal components analysis and factor analysis, to measure how patterns of dietary behavior influence the risk of developing cancer.[14] (The most well-studied dietary pattern is the mediterranean diet.) Based on their dietary pattern score, epidemiologists categorize people into quantiles. To estimate the influence of dietary behavior on risk of cancer, they measure the association between quantiles and the distribution of cancer prevalence (in case-control studies) and cancer incidence (in longitudinal studies). They usually include other variables in their statistical model to account for the other differences between people with and without cancer (confounders). For breast cancer, there is a replicated trend for women with a more "prudent or healthy" diet, i.e. higher in fruits and vegetables, to have a lower risk of cancer.[15] A "drinker dietary pattern" is also associated with higher breast cancer risk, while the association is inconsistent between a more westernized diet and elevated risk of breast cancer. Pickled foods are linked with cancer.
Western pattern diet
Mediterranean diet
Dietary components
Alcohol
Alcohol is associated with an increased risk of a number of cancers.[16] It has been reported that 3.6% of all cancer cases and 3.5% of cancer deaths worldwide are attributable to drinking of alcohol.[17] Breast cancer in women is linked with alcohol intake.[3][18] Alcohol also increases the risk of cancers of the mouth, esophagus, pharynx and larynx,[19] colorectal cancer,[20][21] liver cancer,[22] stomach[23] and ovaries.[24] The International Agency for Research on Cancer (Centre International de Recherche sur le Cancer) of the World Health Organization has classified alcohol as a Group 1 carcinogen. Its evaluation states, "There is sufficient evidence for the carcinogenicity of alcoholic beverages in humans. ... Alcoholic beverages are carcinogenic to humans (Group 1)."[25]
Eggs
Processed and red meat
There is strong evidence that processed meat and red meat intake increases risk of colorectal cancer.[26][27][28] The American Cancer Society in their "Diet and Physical Activity Guideline", stated "evidence that red and processed meats increase cancer risk has existed for decades, and many health organizations recommend limiting or avoiding these foods."[29]
On October 26, 2015, the International Agency for Research on Cancer of the World Health Organization reported that eating processed meat (e.g., bacon, ham, hot dogs, sausages) or red meat was linked to some cancers and classed them as Group 1 (carcinogenic to humans) and Group 2a (probably carcinogenic to humans) carcinogens respectively.[30] There is some evidence that suggests that heme and nitrite are involved in the processes linking red and processed meat intake with colorectal cancer.[30] Heme is present in particular in red meat and nitrite is used as curing salt in many processed meats.
Salted fish
Fiber, fruits and vegetables
There is strong evidence that consumption of dietary fiber reduces risk of colorectal cancer.[31][32][33] Two 2020 meta-analyses found that a high fiber intake was associated with a lower risk of both premenopausal and postmenopausal breast cancers[34] and a higher survival rate in patients with breast cancer.[35]
A 2021 review found that there is moderate-quality evidence 200g of fruit intake per day is associated with a lower risk of breast cancer.[36] Another review found that high total fruit and vegetable consumption are associated with reduced risk of breast cancer.[37]
Pickled vegetables
Flavonoids
Flavonoids (specifically flavonoids such as the catechins) are "the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants."[38] While some studies have suggested flavonoids may have a role in cancer prevention, others have been inconclusive or suggested they may be harmful.[39][40]
Mushrooms
According to Cancer Research UK, "there is currently no evidence that any type of mushroom or mushroom extract can prevent or cure cancer", although research into some species continues.[41]
Dairy products
Whole grains
There is strong evidence that consumption of whole grains decreases risk of colorectal cancer.[32][42][43][44]
Saturated fat
Other
- According to the American Cancer Society, there is no conclusive evidence for an anticancer effect of consuming soy products.[45]
- Green tea consumption has no effect on cancer risk.[46][47][48]
- A 2016 meta-analysis showed that women and men who drank coffee had a lower risk of liver cancer.[9] An umbrella review of meta-analyses found that coffee was associated with a lower risk of liver and endometrial cancer.[49]
- A 2014 systematic review found, "no firm evidence that vitamin D supplementation affects cancer occurrence in predominantly elderly community-dwelling women."[50]
Mechanisms of action
Methionine metabolism
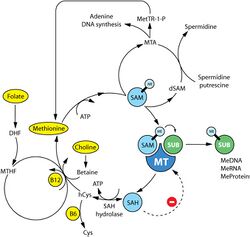
Although numerous cellular mechanisms are involved in food intake, many investigations over the past decades have pointed out defects in the methionine metabolic pathway as cause of carcinogenesis.[51][52] For instance, deficiencies of the main dietary sources of methyl donors, methionine and choline, lead to the formation of liver cancer in rodents.[53][54] Methionine is an essential amino acid that must be provided by dietary intake of proteins or methyl donors (choline and betaine found in beef, eggs and some vegetables). Assimilated methionine is transformed in S-adenosyl methionine (SAM) which is a key metabolite for polyamine synthesis, e.g. spermidine, and cysteine formation (see the figure on the right). Methionine breakdown products are also recycled back into methionine by homocysteine remethylation and methylthioadenosine (MTA) conversion (see the figure on the right). Vitamins B6, B12, folic acid and choline are essential cofactors for these reactions. SAM is the substrate for methylation reactions catalyzed by DNA, RNA and protein methyltransferases.
The products of these reactions are methylated DNA, RNA or proteins and S-adenosylhomocysteine (SAH). SAH has a negative feedback on its own production as an inhibitor of methyltransferase enzymes. Therefore, SAM:SAH ratio directly regulates cellular methylation, whereas levels of vitamins B6, B12, folic acid and choline regulates indirectly the methylation state via the methionine metabolism cycle.[55][56] A near ubiquitous feature of cancer is a maladaption of the methionine metabolic pathway in response to genetic or environmental conditions resulting in depletion of SAM and/or SAM-dependent methylation. Whether it is deficiency in enzymes such as methylthioadenosine phosphorylase, methionine-dependency of cancer cells, high levels of polyamine synthesis in cancer, or induction of cancer through a diet deprived of extrinsic methyl donors or enhanced in methylation inhibitors, tumor formation is strongly correlated with a decrease in levels of SAM in mice, rats and humans.[57][58]
According to a 2012 review, the effect of methionine restriction on cancer has yet to be studied directly in humans and "there is still insufficient knowledge to give reliable nutritional advice".[59]
AMPK
AMPK is thought to be a major element or mechanism in cancer-related effects of diet. It modulates the activity of cellular survival signaling such as mTOR and Akt, leading to cell growth inhibition which is relevant to cancer growth. Targeting AMPK has become a novel strategy for cancer prevention and treatment.[60][61][62] Potential complementary or preventive options under investigation include periods of caloric restriction and AMPK agonists (typically mTOR inhibitors).[63][64][65][66][67][68] However, AMPK can also promote cancer in some[clarification needed] settings.[60][65]
See also
- Alcohol and cancer
- Alcohol and breast cancer
- Acrylamide
- Bovine Meat and Milk Factors
- Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective
- List of ineffective cancer treatments
- List of topics characterized as pseudoscience
- Microplastics ingested through diet
- Zero waste supermarket
References
- ↑ "Cancer is a preventable disease that requires major lifestyle changes". Pharmaceutical Research 25 (9): 2097–2116. September 2008. doi:10.1007/s11095-008-9661-9. PMID 18626751.
- ↑ "Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults". JAMA Internal Medicine 176 (6): 816–825. June 2016. doi:10.1001/jamainternmed.2016.1548. PMID 27183032.
- ↑ 3.0 3.1 3.2 3.3 "Diet and cancer". Swiss Medical Weekly 141: w13250. 9 September 2011. doi:10.4414/smw.2011.13250. PMID 21904992.
- ↑ "Ch. 2: Cancer Etiology § 6 Diet, obesity and physical activity". World Cancer Report 2014. World Health Organization. 2014. pp. 124–33. ISBN 978-92-832-0429-9.
- ↑ "Meat, fish, dairy and cancer risk". wcrf.org. Retrieved 24 April 2023.
- ↑ "Red Meat and Processed Meat Consumption". progressreport.cancer.gov. Retrieved 24 April 2023.
- ↑ "Red Meat (Beef, Pork, Lamb): Increases Risk of Colorectal Cancer". aicr.org. Retrieved 24 April 2023.
- ↑ 8.0 8.1 8.2 "Aetiology of cancer in Asia". Asian Pacific Journal of Cancer Prevention 9 (3): 371–380. 2008. PMID 18990005. http://www.apocpcontrol.org/paper_file/issue_abs/Volume9_No3/371%20Park.pdf.
- ↑ 9.0 9.1 "An updated dose-response meta-analysis of coffee consumption and liver cancer risk". Scientific Reports 6 (1): 37488. December 2016. doi:10.1038/srep37488. PMID 27910873. Bibcode: 2016NatSR...637488Y.
- ↑ "Epidemiology of Stomach Cancer". Cancer Epidemiology. Methods in Molecular Biology. 472. 2009. pp. 467–477. doi:10.1007/978-1-60327-492-0_23. ISBN 978-1-60327-491-3.
- ↑ "Cancer mortality among Japanese Issei and Nisei of California". Cancer 18 (5): 656–664. May 1965. doi:10.1002/1097-0142(196505)18:5<656::AID-CNCR2820180515>3.0.CO;2-3. PMID 14278899.
- ↑ "Preventing Cancer". hsph.harvard.edu. Retrieved 24 April 2023.
- ↑ "[How useful are diets against cancer?]". Deutsche Medizinische Wochenschrift 137 (47): 2417–2422. November 2012. doi:10.1055/s-0032-1327276. PMID 23152069.
- ↑ "Dietary patterns and breast cancer: a review with focus on methodological issues". Nutrition Reviews 67 (6): 297–314. June 2009. doi:10.1111/j.1753-4887.2009.00203.x. PMID 19519672.
- ↑ "Dietary patterns and breast cancer risk: a systematic review and meta-analysis". The American Journal of Clinical Nutrition 91 (5): 1294–1302. May 2010. doi:10.3945/ajcn.2009.28796. PMID 20219961.
- ↑ National Institute on Alcohol Abuse and Alcoholism (NIAAA) (July 1993). "Alcohol and Cancer". Alcohol Alert 21: PH 345. http://pubs.niaaa.nih.gov/publications/aa21.htm.
- ↑ "The burden of cancer attributable to alcohol drinking". International Journal of Cancer 119 (4): 884–887. August 2006. doi:10.1002/ijc.21903. PMID 16557583.
- ↑ "Epidemiology and pathophysiology of alcohol and breast cancer: Update 2012". Alcohol and Alcoholism 47 (3): 204–212. May–June 2012. doi:10.1093/alcalc/ags011. PMID 22459019.
- ↑ "Ch. 4: Food and Drinks §8: Alcoholic drinks". Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. World Cancer Research Fund / American Institute for Cancer Research (AICR) Expert Reports. 2. Washington, DC: AICR. 2007. pp. 157–71. ISBN 978-0-9722522-2-5. http://www.aicr.org/assets/docs/pdf/reports/Second_Expert_Report.pdf#182. Retrieved 2014-08-29.
- ↑ "Alcohol consumption and risk of colon cancer: evidence from the national health and nutrition examination survey I epidemiologic follow-up study". Nutrition and Cancer 50 (2): 111–119. 2004. doi:10.1207/s15327914nc5002_1. PMID 15623458.
- ↑ "Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies". Annals of Internal Medicine 140 (8): 603–613. April 2004. doi:10.7326/0003-4819-140-8-200404200-00007. PMID 15096331. https://cris.maastrichtuniversity.nl/en/publications/c334e177-5e5d-4b7a-87e4-56dad81a520b.
- ↑ "Alcohol in hepatocellular cancer". Clinics in Liver Disease 9 (1): 151–169. February 2005. doi:10.1016/j.cld.2004.10.003. PMID 15763234.
- ↑ "Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: results from a case-control study in Montreal". Cancer Detection and Prevention 32 (5–6): 352–362. 2009. doi:10.1016/j.canep.2009.03.001. PMID 19588541.
- ↑ "Alcohol consumption and the risk of cancer: a meta-analysis". Alcohol Research & Health 25 (4): 263–270. 2001. PMID 11910703. PMC 6705703. http://pubs.niaaa.nih.gov/publications/arh25-4/263-270.htm. Retrieved 2012-11-25.
- ↑ "Ch. 6: Summary of Data Reported and Evaluation §5: Evaluation". Alcohol Drinking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 44. Lyon: International Agency for Research on Cancer (IARC): World Health Organization. 1988. pp. 258–9. ISBN 978-92-832-1244-7. http://monographs.iarc.fr/ENG/Monographs/vol44/mono44-10.pdf.
- ↑ "Does eating processed and red meat cause cancer?" (in en). 25 June 2019. https://www.cancerresearchuk.org/about-cancer/causes-of-cancer/diet-and-cancer/does-eating-processed-and-red-meat-cause-cancer.
- ↑ "Red Meat (Beef, Pork, Lamb): Increases Risk of Colorectal Cancer" (in en-US). https://www.aicr.org/cancer-prevention/food-facts/red-meat-beef-pork-lamb/.
- ↑ "Limit red and processed meat" (in en-US). https://www.wcrf.org/diet-activity-and-cancer/cancer-prevention-recommendations/limit-red-and-processed-meat/.
- ↑ Rock, Cheryl L.; Thomson, Cynthia; Gansler, Ted; Gapstur, Susan M.; McCullough, Marjorie L.; Patel, Alpa V.; Andrews, Kimberly S.; Bandera, Elisa V. et al. (2020). "American Cancer Society guideline for diet and physical activity for cancer prevention". CA 70 (4): 245–271. doi:10.3322/caac.21591. PMID 32515498.
- ↑ 30.0 30.1 Staff (October 26, 2015). "World Health Organization - IARC Monographs evaluate consumption of red meat and processed meat". International Agency for Research on Cancer. http://www.iarc.fr/en/media-centre/pr/2015/pdfs/pr240_E.pdf.
- ↑ "Wholegrains, vegetables, fruit and cancer risk". wcrf.org. Retrieved 13 April 2023.
- ↑ 32.0 32.1 "Wholegrains, vegetables and fruit and the risk of cancer". wcrf.org. Retrieved 13 April 2023.
- ↑ "Levels of evidence for the association between different food groups/items consumption and the risk of various cancer sites: an umbrella review". Int J Food Sci Nutr 73 (7): 861–874. 2022. doi:10.1080/09637486.2022.2103523. PMID 35920747.
- ↑ "Fiber consumption and breast cancer incidence: A systematic review and meta-analysis of prospective studies". Cancer 126 (13): 3061–3075. July 2020. doi:10.1002/cncr.32816. PMID 32249416.
- ↑ "Dietary Fiber and Survival in Women with Breast Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies". Nutrition and Cancer 73 (9): 1570–1580. 2020. doi:10.1080/01635581.2020.1803928. PMID 32795218.
- ↑ "Fruit consumption and multiple health outcomes: An umbrella review". Trends in Food Science & Technology 118: 505–528. 2021. doi:10.1016/j.tifs.2021.09.023. https://www.sciencedirect.com/science/article/abs/pii/S0924224421005471.
- ↑ "Fruit and vegetable consumption and incident breast cancer: a systematic review and meta-analysis of prospective studies". Br J Cancer 125 (2): 284–298. 2021. doi:10.1038/s41416-021-01373-2. PMID 34006925.
- ↑ "Flavonoids: modulators of brain function?". The British Journal of Nutrition 99 E Suppl 1 (E Suppl 1): ES60–ES77. May 2008. doi:10.1017/S0007114508965776. PMID 18503736.
- ↑ "Flavonoids and cancer prevention: a review of the evidence". Journal of Nutrition in Gerontology and Geriatrics 31 (3): 206–238. 2012. doi:10.1080/21551197.2012.702534. PMID 22888839.
- ↑ "Dietary flavonoid for preventing colorectal neoplasms". The Cochrane Database of Systematic Reviews 8 (8): CD009350. August 2012. doi:10.1002/14651858.CD009350.pub2. PMID 22895989.
- ↑ "Mushrooms in cancer treatment § Mushrooms and cancer". Cancer Research UK. 30 January 2013. http://www.cancerresearchuk.org/cancer-help/about-cancer/cancer-questions/mushrooms-in-cancer-treatment#mushrooms.
- ↑ "The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions". The Journal of Nutrition 150 (4): 663–671. 2020. doi:10.1093/jn/nxz268. PMID 31758189.
- ↑ "An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites". Nature Communications 12 (1): 4579. 2021. doi:10.1038/s41467-021-24861-8. PMID 34321471. Bibcode: 2021NatCo..12.4579P.
- ↑ "Association of whole grains intake and the risk of digestive tract cancer: a systematic review and meta-analysis". Nutrition Journal 19 (1): 52. 2021. doi:10.1186/s12937-020-00556-6. PMID 32493399.
- ↑ "Soybean". American Cancer Society. 17 January 2013. http://www.cancer.org/treatment/treatmentsandsideeffects/complementaryandalternativemedicine/dietandnutrition/soybean.
- ↑ "Green tea (Camellia sinensis) for the prevention of cancer". The Cochrane Database of Systematic Reviews 3 (11): CD005004. March 2020. doi:10.1002/14651858.CD005004.pub3. PMID 32118296.
- ↑ "Green Tea". American Cancer Society. 4 May 2012. http://www.cancer.org/treatment/treatmentsandsideeffects/complementaryandalternativemedicine/herbsvitaminsandminerals/green-tea.
- ↑ "Tea Drinking and Risk of Cancer Incidence: A Meta-Analysis of Prospective Cohort Studies and Evidence Evaluation". Advances in Nutrition 12 (2): 402–412. March 2021. doi:10.1093/advances/nmaa117. PMID 33002099.
- ↑ "Coffee drinking and cancer risk: an umbrella review of meta-analyses of observational studies". BMC Cancer 20 (1): 101. February 2020. doi:10.1186/s12885-020-6561-9. PMID 32024485.
- ↑ "Vitamin D supplementation for prevention of cancer in adults". The Cochrane Database of Systematic Reviews 6 (6): CD007469. June 2014. doi:10.1002/14651858.CD007469.pub2. PMID 24953955.
- ↑ "Hepatocarcinogenesis in rats fed methyl-deficient, amino acid-defined diets". Carcinogenesis 4 (12): 1619–1629. December 1983. doi:10.1093/carcin/4.12.1619. PMID 6317218.
- ↑ "The induction of liver cancer by dietary deficiency of choline and methionine without added carcinogens". Carcinogenesis 5 (10): 1367–1370. October 1984. doi:10.1093/carcin/5.10.1367. PMID 6488458.
- ↑ "A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice". Carcinogenesis 22 (11): 1871–1875. November 2001. doi:10.1093/carcin/22.11.1871. PMID 11698351.
- ↑ "Male rats fed methyl- and folate-deficient diets with or without niacin develop hepatic carcinomas associated with decreased tissue NAD concentrations and altered poly(ADP-ribose) polymerase activity". The Journal of Nutrition 127 (1): 30–36. January 1997. doi:10.1093/jn/127.1.30. PMID 9040540.
- ↑ "Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice". The Journal of Nutrition 131 (11): 2811–2818. November 2001. doi:10.1093/jn/131.11.2811. PMID 11694601.
- ↑ "Blood determinations of S-adenosylmethionine, S-adenosylhomocysteine, and homocysteine: correlations with diet". Cancer Epidemiology, Biomarkers & Prevention 10 (6): 649–655. June 2001. PMID 11401915. http://cebp.aacrjournals.org/cgi/pmidlookup?view=long&pmid=11401915.
- ↑ "Beneficial role for folate in the prevention of colorectal and breast cancer". European Journal of Nutrition 40 (3): 98–105. June 2001. doi:10.1007/PL00007387. PMID 11697447.
- ↑ "Genetic effects of methylation diets". Annual Review of Nutrition 22: 255–282. 2002. doi:10.1146/annurev.nutr.22.010402.102932. PMID 12055346.
- ↑ "A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension". Cancer Treatment Reviews 38 (6): 726–736. October 2012. doi:10.1016/j.ctrv.2012.01.004. PMID 22342103.
- ↑ 60.0 60.1 Wang, Zhiyu; Wang, Neng; Liu, Pengxi; Xie, Xiaoming (2016). "AMPK and Cancer" (in en). AMP-activated Protein Kinase. Experientia Supplementum. 107. Springer International Publishing. 203–226. doi:10.1007/978-3-319-43589-3_9. ISBN 978-3-319-43587-9.
- ↑ Carling, David (April 2017). "AMPK signalling in health and disease". Current Opinion in Cell Biology 45: 31–37. doi:10.1016/j.ceb.2017.01.005. PMID 28232179.
- ↑ Li, Jin; Zhong, Liping; Wang, Fengzhong; Zhu, Haibo (May 2017). "Dissecting the role of AMP-activated protein kinase in human diseases". Acta Pharmaceutica Sinica B 7 (3): 249–259. doi:10.1016/j.apsb.2016.12.003. PMID 28540163.
- ↑ Yung, Mingo M.H.; Ngan, Hextan Y.S.; Chan, David W. (1 April 2016). "Targeting AMPK signaling in combating ovarian cancers: opportunities and challenges". Acta Biochimica et Biophysica Sinica 48 (4): 301–317. doi:10.1093/abbs/gmv128. PMID 26764240.
- ↑ Meynet, Ophélie; Ricci, Jean-Ehrland (August 2014). "Caloric restriction and cancer: molecular mechanisms and clinical implications". Trends in Molecular Medicine 20 (8): 419–427. doi:10.1016/j.molmed.2014.05.001. ISSN 1471-499X. PMID 24916302.
- ↑ 65.0 65.1 Fay, Judith R.; Steele, Vernon; Crowell, James A. (1 April 2009). "Energy Homeostasis and Cancer Prevention: The AMP-Activated Protein Kinase". Cancer Prevention Research 2 (4): 301–309. doi:10.1158/1940-6207.CAPR-08-0166. PMID 19336731.
- ↑ Skuli, Sarah J.; Alomari, Safwan; Gaitsch, Hallie; Bakayoko, A'ishah; Skuli, Nicolas; Tyler, Betty M. (19 May 2022). "Metformin and Cancer, an Ambiguanidous Relationship". Pharmaceuticals 15 (5): 626. doi:10.3390/ph15050626. PMID 35631452.
- ↑ Ingram, Donald K.; Roth, George S. (June 2021). "Glycolytic inhibition: an effective strategy for developing calorie restriction mimetics". GeroScience 43 (3): 1159–1169. doi:10.1007/s11357-020-00298-7. PMID 33184758.
- ↑ Guigas, Bruno; Viollet, Benoit (2016). "Targeting AMPK: From Ancient Drugs to New Small-Molecule Activators". AMP-activated Protein Kinase. Experientia Supplementum. 107. 327–350. doi:10.1007/978-3-319-43589-3_13. ISBN 978-3-319-43587-9.
External links
- "Diet, healthy eating and cancer". Cancer Research UK. 2013-08-22. http://info.cancerresearchuk.org/healthyliving/dietandhealthyeating/?a=5441.
- "EPIC (European Prospective Investigation into Cancer and Nutrition) Study". International Agency for Research on Cancer: World Health Organization. http://epic.iarc.fr/.
{{Navbox
| name = Tumors | title = Overview of tumors, cancer and oncology (C00–D48, 140–239) | state = autocollapse | listclass = hlist
| group1 = Conditions
| list1 =
| Benign tumors | |
|---|---|
| Malignant progression | |
| Topography | |
| Histology | |
| Other |
| group2 = Staging/grading | list2 =
| group3 = Carcinogenesis | list3 =
- Cancer cell
- Carcinogen
- [[Biology:Tumor suppressor Tumor suppressor genes/oncogenes
- Clonally transmissible cancer
- Oncovirus
- Carcinogenic bacteria
| group4 = Misc. | list4 =
}}
 |



