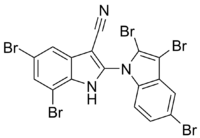
Chemistry:Aetokthonotoxin
| |
| Names | |
|---|---|
| IUPAC name
5,7-Dibromo-2-(2,3,5-tribromoindol-1-yl)-1H-indole-3-carbonitrile
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| |
| |
| Properties | |
| C17H6Br5N3 | |
| Molar mass | 651.776 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
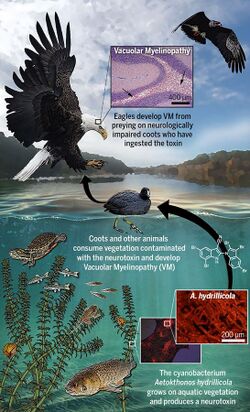
Aetokthonotoxin (AETX), colloquially known as eagle toxin, is a chemical compound that was identified in 2021 as the cyanobacterial neurotoxin causing vacuolar myelinopathy (VM) in eagles in North America.[1] As the biosynthesis of aetokthonotoxin depends on the availability of bromide in freshwater systems and requires an interplay between the toxin-producing cyanobacterium Aetokthonos hydrillicola and the host plant it epiphytically grows on (most importantly hydrilla), it took more than 25 years to identify aetokthonotoxin as the VM-inducing toxin after the disease has first been diagnosed in bald eagles in 1994.[2] The toxin cascades through the food-chain: Among other animals, it affects fish and waterfowl such as coots or ducks which feed on hydrilla colonized with the cyanobacterium. Aetokthonotoxin is transmitted to raptors, such as the bald eagle, that prey on these affected animals.[3] The total synthesis of AETX has been achieved in 2021,[4] the enzymatic functions of the 5 enzymes involved in AETX biosynthesis were described in 2022.[5]
Biosynthesis
The biosynthesis of AETX and the functions of the enzymes AetA, AetB, AetD, AetE, and AetF were described in 2022.[5] AetF, a FAD-dependent halogenase, brominates L-tryptophan at the C5 position. The 5-bromo-L-tryptophan can then undergo two separate reactions. One route involves a second bromination by AetF at C7 to yield 5,7-dibromo-L-tryptophan. This molecule then goes on to react with AetD, an iron-dependent nitrile synthase, to form dibromo-indole-3-carbonitrile. The second route for 5-bromo-L-tryptophan involves the tryptophanase AetE, which converts the 5-bromo-L-tryptophan into 5-bromoindole. 5-bromoindole can then go on to react with a different FAD-dependent halogenase AetA to form 2,3,5-tribromoindole. 2,3,5-tribromoindole and dibromo-indole-3-carbonitrile then undergo biaryl coupling facilitated by the cytochrome P-450 AetB to form AETX.
See also
References
- ↑ Breinlinger, Steffen; Phillips, Tabitha J.; Haram, Brigette N.; Mareš, Jan; Yerena, José A. Martínez; Hrouzek, Pavel; Sobotka, Roman; Henderson, W. Matthew et al. (2021-03-26). "Hunting the eagle killer: A cyanobacterial neurotoxin causes vacuolar myelinopathy" (in en). Science 371 (6536): eaax9050. doi:10.1126/science.aax9050. ISSN 0036-8075. PMID 33766860.
- ↑ "Avian vacuolar myelinopathy". USGS National Wildlife Health Center. http://www.nwhc.usgs.gov/disease_information/avian_vacuolar_myelinopathy/. Retrieved 24 October 2013.
- ↑ Birrenkott, A. H.; S. B Wilde; J. J. Hains; J. R. Fisher; T. M. Murphy; C. P. Hope; P. G. Parnell; W. W. Bowerman (2004). "Establishing a food-chain link between aquatic plant material and avian vacuolar myelinopathy in mallards (Anas platyrhynchos)". Journal of Wildlife Diseases 40 (3): 485–492. doi:10.7589/0090-3558-40.3.485. PMID 15465716.
- ↑ Manuel G. Ricardo, Markus Schwark, Dayma Llanes, Timo H. J. Niedermeyer, Bernhard Westermann (2021-06-03), "Total Synthesis of Aetokthonotoxin, the Cyanobacterial Neurotoxin Causing Vacuolar Myelinopathy" (in German), Chemistry – A European Journal 27 (47): 12032–12035, doi:10.1002/chem.202101848, PMID 34081364
- ↑ 5.0 5.1 Adak, Sanjoy; Lukowski, April L.; Schäfer, Rebecca J. B.; Moore, Bradley S. (2022-02-10). "From Tryptophan to Toxin: Nature's Convergent Biosynthetic Strategy to Aetokthonotoxin". Journal of the American Chemical Society (American Chemical Society (ACS)) 144 (7): 2861–2866. doi:10.1021/jacs.1c12778. ISSN 0002-7863. PMID 35142504.
 |