Chemistry:Oenanthotoxin
| |
| Names | |
|---|---|
| Preferred IUPAC name
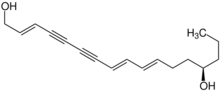
(2E,8E,10E,14R)-Heptadeca-2,8,10-triene-4,6-diyne-1,14-diol | |
| Other names
Enanthotoxin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C17H22O2 | |
| Molar mass | 258.361 g·mol−1 |
| Melting point | 86 °C (187 °F; 359 K) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
0.58 mg/kg for mice |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oenanthotoxin is a toxin extracted from hemlock water-dropwort (Oenanthe crocata) and other plants of the genus Oenanthe. It is a central nervous system poison, and acts as a noncompetitive antagonist of the neurotransmitter gamma-aminobutyric acid.[1] A case has been made for the presence of this toxin in local Oenanthe species playing a causative role in euthanasia in ancient Sardinia.[2][3] It was crystallized in 1949 by Clarke and co-workers.[4] It is structurally closely related to the toxins cicutoxin[5] and carotatoxin.[6][7] Oenanthotoxin is a C17 polyacetylene isomer of cicutoxin.
Occurrence
Oenanthotoxin concentration in plants is dependent on seasonal changes and geographical location, the most is present during late winter and early spring.[8] Contrary to most poisonous plants that contain bitter tastes or burning sensations, the water dropwort has a rather sweet and pleasant taste and odor.[9] Water dropwort is characterized by a yellow liquid that changes color due to air exposure.[1][9] The roots are the most toxic part, although the entire plant contains poisonous properties.[8][10]
History and culture
The discovery and use of plants containing oenanthotoxin predates Socrates and Homer and its first use as a poison is thought to have been implemented between 1800 BC and 800 BC in Pre-Roman Sardinia.[9][11] In Ancient Sardinia, it was considered to be a humane form of euthanasia. Elderly people who were unable to care for themselves were given water dropwort and dropped from a high rock to ensure death.[9][11] It is also believed that Socrates ingested the plant when executed.[12]
A common symptom of oenanthotoxin is risus sardonicus, better known as the Sardonic Grin, coined by Homer in the 8th century BC, due to the victim's rigid smile after ingestion.
Furthermore, as a muscle relaxant, it is believed to have cosmetic botox-like properties in small amounts.[11]
Mechanism of action
Although oenanthotoxin is a relatively well known poison, its mechanism of action is not entirely understood. However, there is evidence that its mechanism of action is similar to that of cicutoxin.
Oenanthotoxin is part of a group of C17 conjugated polyacetylenes that act as noncompetitive gamma-aminobutyric acid (GABA) inhibitors in the central nervous system (CNS). GABA binds to the beta-domain of the GABAA receptor in the central nervous system and activates the receptor increasing chloride ion flow across the membrane and inhibiting the neuron.[1] When oenanthotoxin is introduced to the body, it non-competitively binds to the same beta-domain receptor as GABA and prevents normal inhibitory function. Binding to the same receptor, oenanthotoxin blocks the chloride channel, allowing excessive excitation to occur. This, blocking GABAergic responses, causes hyperactivity in the neurons, resulting in convulsions, and seizures.[9]
Symptoms
While oenanthotoxin is extremely dangerous and toxic (LD50 = 0.58 mg/kg for mice),[1] there have been numerous case studies documenting the common symptoms including: convulsions, seizures, nausea, diarrhea, tachycardia, mydriasis, rhabdomyolysis, renal failure, respiratory impairment, and cardiac dysrhythmias.[1][8][9]
Below is a comprehensive table listing the recorded symptoms caused oenanthotoxin within each system in the body Oenanthe crocata:[1]
| Organ system | Symptoms |
|---|---|
| Neurological | slurred speech, dizziness, paresthesia, delirium, ataxia, coma, seizures, trismus, hyperreflexia, opisthotonus, spasms, cerebral edema, status epilepticus |
| Gastrointestinal | nausea, vomiting, salivation, abdominal pain |
| Respiratory | congestion, distress, depression, airway obstruction, arrest, apnea |
| Cardivascular | tachycardia, brachycardia, hypertension, hypotension, cardiac dysrhythmias, cardiac arrest |
| Renal | glycosuria, proteinuria, hematuria, oliguria, myoglobinuria, acute renal failure |
| Musculoskeletal | weakness, muscle spasms, muscle rigidity, rhabdomyolysis |
| Metabolic | elevated temperature, liver dysfunction, hypokalemia, lactic dehydrogenase, disseminating (intravascular, coagulation), metabolic acidosis, azotemia |
| Occular | mydriasis |
| Dermal | diaphoresis, cyanosis, flushed face |
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Schep, L. J.; Slaughter, R. J.; Becket, G.; Beasley, D. M. G. (2009). "Poisoning due to Water Hemlock". Clinical Toxicology 47 (4): 270–278. doi:10.1080/15563650902904332. PMID 19514873.
- ↑ Appendino, G.; Pollastro, F.; Verotta, L.; Ballero, M.; Romano, A.; Wyrembek, P.; Szczuraszek, K.; Mozrzymas, J. W. et al. (2009). "Polyacetylenes From Sardinian Oenanthe fistulosa: A Molecular Clue to risus sardonicus". Journal of Natural Products 72 (5): 962–965. doi:10.1021/np8007717. PMID 19245244.
- ↑ Choi, C. Q.; Harmon, K.; Matson, J. (August 2009). "News Scan Briefs: Killer Smile". Scientific American. doi:10.1038/scientificamerican0809-26b. http://www.scientificamerican.com/article.cfm?id=in-brief-aug-09.
- ↑ E. G. C. Clarke, D. E. Kidder and W. D. Robertson (1949) J. Pharm. Pharmacol. 1 377-381
- ↑ Anet, E. F. L. J.; Lythgoe, B.; Silk, M. H.; Trippett, S. (1953). "Oenanthotoxin and Cicutoxin. Isolation and Structures". Journal of the Chemical Society 1953: 309–322. doi:10.1039/JR9530000309.
- ↑ King, L. A.; Lewis, M. J.; Parry, D.; Twitchett, P. J.; Kilner, E. A. (1985). "Identification of Oenanthotoxin and Related Compounds in Hemlock Water Dropwort Poisoning". Human Toxicology 4 (4): 355–364. doi:10.1177/096032718500400401. PMID 4018815.
- ↑ Anet, E. F. L. J.; Lythgoe, B.; Silk, M. H.; Trippett, S. (1952). "The Chemistry of Oenanthotoxin and Cicutoxin". Chemistry and Industry 31: 757–758.
- ↑ 8.0 8.1 8.2 "Information Sheet: 31 Hemlock Water Dropwort (Oenanthe crocata)". Centre for Aquatic Plant Management. http://www.ceh.ac.uk/sci_programmes/documents/31oenanthecrocata.pdf.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Appendino, G.; Pollastro, F.; Verotta, L. (2009), "Polyacetylenes from Sardinian Oenanthe Fistulosa: A Molecular Clue to risus sardonicus", J. Nat. Prod. 72 (5): 962–965, doi:10.1021/np8007717, PMID 19245244
- ↑ Egdahl, A. (1911). "A case of poisoning due to eating poison hemlock (Cicuta maculata) with a review of reported cases". Arch Intern Med 7 (3): 348–356. doi:10.1001/archinte.1911.00060030061002. https://zenodo.org/record/1423309.
- ↑ 11.0 11.1 11.2 Owen, James. "Ancient Death-Smile Potion Decoded?". Journal of Natural Products. http://news.nationalgeographic.com/news/2009/06/090602-smiling-death-potion.html.
- ↑ Bletchly, Rachael. "Killers in your garden; Beware these poison plants". Gale, Cengage Learning. http://www.thefreelibrary.com/Killers+in+your+garden%3b+Beware+these+poison+plants-a0389234899.
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}}
 |
