Chemistry:Penitrem A
| |
| Names | |
|---|---|
| Other names
Tremortin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
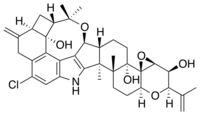
| C37H44ClNO6 | |
| Molar mass | 633.20136 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Penitrem A (tremortin) is an indole-diterpenoid mycotoxin produced by certain species of Aspergillus, Claviceps, and Penicillium, which can be found growing on various plant species such as ryegrass.[1] Penitrem A is one of many secondary metabolites following the synthesis of paxilline in Penicillium crostosum.[2] Penitrem A poisoning in humans and animals usually occurs through the consumption of contaminated foods by mycotoxin-producing species, which is then distributed through the body by the bloodstream.[2] It bypasses the blood-brain barrier to exert its toxicological effects on the central nervous system.[2] In humans, penitrem A poisoning has been associated with severe tremors, hyperthermia, nausea/vomiting, diplopia, and bloody diarrhea.[2] In animals, symptoms of penitrem A poisoning has been associated with symptoms ranging from tremors, seizures, and hyperthermia to ataxia and nystagmus.[2]
Roquefortine C has been commonly detected in documented cases of penitrem A poisoning, making it a possible biomarker for diagnoses.[3]
Mechanism of action
Penitrem A impairs GABAergic amino acid neurotransmission and antagonizes high-conductance Ca2+-activated potassium channels in both humans and animals.[4] Impairment of the GABAergic amino acid neurotransmission comes with the spontaneous release of the excitatory amino acids glutamate and aspartate as well as the inhibitory neurotransmitter γ-aminobutyric acid (GABA).[4] The sudden release of these neurotransmitters results in imbalanced GABAergic signalling, which gives rise to neurological disorders such as the tremors associated with penitrem A poisoning.[4]
Penitrem A also induces the production of reactive oxygen species (ROS) in the neutrophil granulocytes of humans and animals.[2] Increased ROS production results in tissue damage in the brain and other afflicted organs as well as hemorrhages in acute poisonings.[2]
Synthesis
In Penicillium crustosum, synthesis of penitrem A and other secondary metabolites follows the synthesis of paxilline.[5] Synthesis of penitrem A involves six oxidative-transformation enzymes (four cytochrome P450 monooxygenases and two flavin adenine dinucleotide (FAD)-dependent monooxygenases), two acetyltransferases, one oxidoreductase, and one prenyltransferase.[5] These enzymes are encoded by a cluster of genes used in paxilline synthesis and penitrem A-F synthesis.[5] The pathway is described below:
- Oxidoreductase catalyzes the reduction of paxilline's ketone and also adds a dimethylallyl group to its aromatic ring.[5]
- Acetyltransferases catalyze the removal of the intermediate's lower right-hand hydroxyl group and reduce of one of the nearby methyl groups to a methylene group.[5]
- Oxidative-transformation enzyme catalyzes the addition of a hydroxyl group to the intermediate's dimethylallyl group. The dimethylallyl's double bond migrates down one carbon.[5]
- Prenyltransferase catalyzes the formation of a dimethyl-cyclopentane and a cyclobutane using the intermediate's aromatic ring-alcohol group.[5]
- Oxidative-transformation enzyme catalyzes the formation of a methylenecyclohexane using the intermediate's dimethyl-cyclopentane, forming secopenitrem D.[5]
- Oxidative-transformation enzyme catalyzes the formation of a cyclooctane using cyclobutane's alcohol group and the carbon joining secopenitrem D's cyclohexane and cyclopentane, forming penitrem D.[5]
- Oxidative-transformation enzyme catalyzes the addition a chlorine atom at penitrem D's aromatic ring, forming penitrem C.[5]
- Oxidative-transformation enzyme catalyzes the formation of an epoxide ring at penitrem C's oxane-double bond, forming penitrem F.[5]
- Oxidative-transformation enzyme catalyzes the addition of a hydroxyl group at the carbon joining penitrem F's methylenecyclohexane and cyclobutane, forming penitrem A.[5]
See also
References
- ↑ Walter, Sean L. (2002). "Acute penitrem A and roquefortine poisoning in a dog". The Canadian Veterinary Journal 43 (5): 372–374. ISSN 0008-5286. PMID 12001505.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Berntsen, H.F; Bogen, I.L; Wigestrand, M.B; Fonnum, F; Walaas, S.I; Moldes-Anaya, A (2017-12-01). "The fungal neurotoxin penitrem A induces the production of reactive oxygen species in human neutrophils at submicromolar concentrations" (in en). Toxicology 392: 64–70. doi:10.1016/j.tox.2017.10.008. ISSN 0300-483X. PMID 29037868.
- ↑ Tiwary, AK (March 2009). "Using roquefortine C as a biomarker for penitrem A intoxication". Journal of Veterinary Diagnostic Investigation 21 (2): 237–239. doi:10.1177/104063870902100210. PMID 19286504.
- ↑ 4.0 4.1 4.2 Moldes-Anaya, Angel S; Fonnum, Frode; Eriksen, Gunnar S; Rundberget, Thomas; Walaas, S. Ivar; Wigestrand, Mattis B (2011-12-01). "In vitro neuropharmacological evaluation of penitrem-induced tremorgenic syndromes: Importance of the GABAergic system" (in en). Neurochemistry International 59 (7): 1074–1081. doi:10.1016/j.neuint.2011.08.014. ISSN 0197-0186. PMID 21924313.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 Liu, Chengwei; Tagami, Koichi; Minami, Atsushi; Matsumoto, Tomoyuki; Frisvad, Jens Christian; Suzuki, Hideyuki; Ishikawa, Jun; Gomi, Katsuya et al. (2015-04-01). "Reconstitution of Biosynthetic Machinery for the Synthesis of the Highly Elaborated Indole Diterpene Penitrem" (in en). Angewandte Chemie International Edition 54 (19): 5748–5752. doi:10.1002/anie.201501072. ISSN 1433-7851. PMID 25831977.
 |
